Difference between revisions of "ZapA"
| Line 1: | Line 1: | ||
| − | * '''Description:''' positive modulator of Z ring assembly and stability <br/><br/> | + | * '''Description:''' positive modulator of [[FtsZ]] Z ring assembly and stability <br/><br/> |
{| align="right" border="1" cellpadding="2" | {| align="right" border="1" cellpadding="2" | ||
| Line 70: | Line 70: | ||
* '''Effectors of protein activity:''' | * '''Effectors of protein activity:''' | ||
| − | * '''Interactions:''' forms a dimer through its extensive coiled-coil region | + | * '''Interactions:''' forms a dimer through its extensive coiled-coil region, [[FtsZ]]-[[ZapA]] {{PubMed|19843223}} |
* '''Localization:''' cell membrane (according to Swiss-Prot) | * '''Localization:''' cell membrane (according to Swiss-Prot) | ||
| Line 121: | Line 121: | ||
==Original Publications== | ==Original Publications== | ||
| − | <pubmed>15288790,12368265,,18588879, 19429628 </pubmed> | + | <pubmed>15288790,12368265,,18588879, 19429628 19843223 </pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 13:12, 22 October 2009
- Description: positive modulator of FtsZ Z ring assembly and stability
| Gene name | zapA |
| Synonyms | yshA |
| Essential | no |
| Product | Z-ring-associated protein |
| Function | control of Z-ring formation |
| MW, pI | 9 kDa, 7.17 |
| Gene length, protein length | 255 bp, 85 aa |
| Immediate neighbours | yshB, rnhC |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 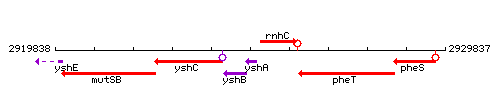
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU28610
Phenotypes of a mutant
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: Type 2 subfamily (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization: cell membrane (according to Swiss-Prot)
Database entries
- Structure:
- UniProt: P94542
- KEGG entry: [2]
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
David W Adams, Jeff Errington
Bacterial cell division: assembly, maintenance and disassembly of the Z ring.
Nat Rev Microbiol: 2009, 7(9);642-53
[PubMed:19680248]
[WorldCat.org]
[DOI]
(I p)
Original Publications
Leigh G Monahan, Andrew Robinson, Elizabeth J Harry
Lateral FtsZ association and the assembly of the cytokinetic Z ring in bacteria.
Mol Microbiol: 2009, 74(4);1004-17
[PubMed:19843223]
[WorldCat.org]
[DOI]
(I p)
Pamela Gamba, Jan-Willem Veening, Nigel J Saunders, Leendert W Hamoen, Richard A Daniel
Two-step assembly dynamics of the Bacillus subtilis divisome.
J Bacteriol: 2009, 191(13);4186-94
[PubMed:19429628]
[WorldCat.org]
[DOI]
(I p)
Dirk-Jan Scheffers
The effect of MinC on FtsZ polymerization is pH dependent and can be counteracted by ZapA.
FEBS Lett: 2008, 582(17);2601-8
[PubMed:18588879]
[WorldCat.org]
[DOI]
(P p)
Harry H Low, Martin C Moncrieffe, Jan Löwe
The crystal structure of ZapA and its modulation of FtsZ polymerisation.
J Mol Biol: 2004, 341(3);839-52
[PubMed:15288790]
[WorldCat.org]
[DOI]
(P p)
Frederico J Gueiros-Filho, Richard Losick
A widely conserved bacterial cell division protein that promotes assembly of the tubulin-like protein FtsZ.
Genes Dev: 2002, 16(19);2544-56
[PubMed:12368265]
[WorldCat.org]
[DOI]
(P p)