Difference between revisions of "YwlE"
(→References) |
|||
| Line 125: | Line 125: | ||
**''ywlE::tet'' available from [[Ulf Gerth]]'s lab | **''ywlE::tet'' available from [[Ulf Gerth]]'s lab | ||
**''ywlE::spec'' available from [[Ulf Gerth]]'s lab | **''ywlE::spec'' available from [[Ulf Gerth]]'s lab | ||
| − | **GP1458 (''ywlE''::''aphA3''), available in [[Jörg Stülke]]'s lab | + | ** GP1458 (''ywlE''::''aphA3''), available in [[Jörg Stülke]]'s lab |
| − | **GP1459 (''ywlE''::''tet''), available in [[Jörg Stülke]]'s | + | ** GP1459 (''ywlE''::''tet''), available in [[Jörg Stülke]]'s and [[Fabian Commichau]]'s labs {{PubMed|25610436}} |
* '''Expression vector:''' for expression, purification in E. coli with N-terminal His-tag, pRSETA available in [[Ulf Gerth]]'s lab | * '''Expression vector:''' for expression, purification in E. coli with N-terminal His-tag, pRSETA available in [[Ulf Gerth]]'s lab | ||
| Line 140: | Line 140: | ||
=Labs working on this gene/protein= | =Labs working on this gene/protein= | ||
* [[Ulf Gerth]], Greifswald, Germany | * [[Ulf Gerth]], Greifswald, Germany | ||
| − | |||
* [[Fabian Commichau]] Göttingen, Germany | * [[Fabian Commichau]] Göttingen, Germany | ||
| Line 147: | Line 146: | ||
=References= | =References= | ||
| − | <pubmed>16452434,15866923,15995210,19678837 21622759, 20852588 22517742 23770242 23838530 24263382 24825175 </pubmed> | + | <pubmed>16452434,15866923,15995210,19678837 21622759, 20852588 22517742 23770242 23838530 24263382 24825175 25610436</pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 09:06, 23 January 2015
- Description: protein arginine phosphatase
| Gene name | ywlE |
| Synonyms | ipc-31d |
| Essential | no |
| Product | protein arginine phosphatase |
| Function | dephosphorylation of McsB |
| Gene expression levels in SubtiExpress: ywlE | |
| MW, pI | 16 kDa, 6.681 |
| Gene length, protein length | 450 bp, 150 aa |
| Immediate neighbours | ywlF, ywlD |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 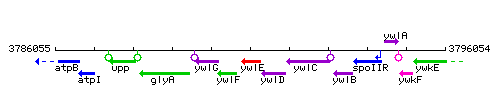
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU36930
Phenotypes of a mutant
Database entries
- BsubCyc: BSU36930
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: Protein arginine phosphate + H2O = protein arginine + phosphate PubMed
- Protein family:
- Paralogous protein(s): YfkJ
Extended information on the protein
- Kinetic information:
- Modification:
- Effectors of protein activity:
- Localization: weak oligomerization, results in inactivation of YwlE PubMed
Database entries
- BsubCyc: BSU36930
- Structure: 1ZGG
- UniProt: P39155
- KEGG entry: [2]
- E.C. number: 3.1.3.48
Additional information
Expression and regulation
- Operon:
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- ywlE::aphA3 available from Ulf Gerth's lab
- ywlE::tet available from Ulf Gerth's lab
- ywlE::spec available from Ulf Gerth's lab
- GP1458 (ywlE::aphA3), available in Jörg Stülke's lab
- GP1459 (ywlE::tet), available in Jörg Stülke's and Fabian Commichau's labs PubMed
- Expression vector: for expression, purification in E. coli with N-terminal His-tag, pRSETA available in Ulf Gerth's lab
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody: available in Ulf Gerth's lab
Labs working on this gene/protein
- Ulf Gerth, Greifswald, Germany
- Fabian Commichau Göttingen, Germany
Your additional remarks
References
Lorena Stannek, Katrin Gunka, Rachel A Care, Ulf Gerth, Fabian M Commichau
Factors that mediate and prevent degradation of the inactive and unstable GudB protein in Bacillus subtilis.
Front Microbiol: 2014, 5;758
[PubMed:25610436]
[WorldCat.org]
[DOI]
(P e)
Débora Broch Trentini, Jakob Fuhrmann, Karl Mechtler, Tim Clausen
Chasing Phosphoarginine Proteins: Development of a Selective Enrichment Method Using a Phosphatase Trap.
Mol Cell Proteomics: 2014, 13(8);1953-64
[PubMed:24825175]
[WorldCat.org]
[DOI]
(I p)
Andreas Schmidt, Débora Broch Trentini, Silvia Spiess, Jakob Fuhrmann, Gustav Ammerer, Karl Mechtler, Tim Clausen
Quantitative phosphoproteomics reveals the role of protein arginine phosphorylation in the bacterial stress response.
Mol Cell Proteomics: 2014, 13(2);537-50
[PubMed:24263382]
[WorldCat.org]
[DOI]
(I p)
Jakob Fuhrmann, Venkataraman Subramanian, Paul R Thompson
Targeting the arginine phosphatase YwlE with a catalytic redox-based inhibitor.
ACS Chem Biol: 2013, 8(9);2024-32
[PubMed:23838530]
[WorldCat.org]
[DOI]
(I p)
Jakob Fuhrmann, Beata Mierzwa, Débora B Trentini, Silvia Spiess, Anita Lehner, Emmanuelle Charpentier, Tim Clausen
Structural basis for recognizing phosphoarginine and evolving residue-specific protein phosphatases in gram-positive bacteria.
Cell Rep: 2013, 3(6);1832-9
[PubMed:23770242]
[WorldCat.org]
[DOI]
(I p)
Alexander K W Elsholz, Kürsad Turgay, Stephan Michalik, Bernd Hessling, Katrin Gronau, Dan Oertel, Ulrike Mäder, Jörg Bernhardt, Dörte Becher, Michael Hecker, Ulf Gerth
Global impact of protein arginine phosphorylation on the physiology of Bacillus subtilis.
Proc Natl Acad Sci U S A: 2012, 109(19);7451-6
[PubMed:22517742]
[WorldCat.org]
[DOI]
(I p)
A K W Elsholz, K Hempel, S Michalik, K Gronau, D Becher, M Hecker, U Gerth
Activity control of the ClpC adaptor McsB in Bacillus subtilis.
J Bacteriol: 2011, 193(15);3887-93
[PubMed:21622759]
[WorldCat.org]
[DOI]
(I p)
Alexander K W Elsholz, Stephan Michalik, Daniela Zühlke, Michael Hecker, Ulf Gerth
CtsR, the Gram-positive master regulator of protein quality control, feels the heat.
EMBO J: 2010, 29(21);3621-9
[PubMed:20852588]
[WorldCat.org]
[DOI]
(I p)
Jascha Blobel, Pau Bernadó, Huimin Xu, Changwen Jin, Miquel Pons
Weak oligomerization of low-molecular-weight protein tyrosine phosphatase is conserved from mammals to bacteria.
FEBS J: 2009, 276(16);4346-57
[PubMed:19678837]
[WorldCat.org]
[DOI]
(I p)
Huimin Xu, Bin Xia, Changwen Jin
Solution structure of a low-molecular-weight protein tyrosine phosphatase from Bacillus subtilis.
J Bacteriol: 2006, 188(4);1509-17
[PubMed:16452434]
[WorldCat.org]
[DOI]
(P p)
Lucia Musumeci, Cristina Bongiorni, Lutz Tautz, Robert A Edwards, Andrei Osterman, Marta Perego, Tomas Mustelin, Nunzio Bottini
Low-molecular-weight protein tyrosine phosphatases of Bacillus subtilis.
J Bacteriol: 2005, 187(14);4945-56
[PubMed:15995210]
[WorldCat.org]
[DOI]
(P p)
Ivan Mijakovic, Lucia Musumeci, Lutz Tautz, Dina Petranovic, Robert A Edwards, Peter Ruhdal Jensen, Tomas Mustelin, Josef Deutscher, Nunzio Bottini
In vitro characterization of the Bacillus subtilis protein tyrosine phosphatase YwqE.
J Bacteriol: 2005, 187(10);3384-90
[PubMed:15866923]
[WorldCat.org]
[DOI]
(P p)