Difference between revisions of "YvyD"
| Line 35: | Line 35: | ||
<br/><br/><br/><br/> | <br/><br/><br/><br/> | ||
<br/><br/><br/><br/> | <br/><br/><br/><br/> | ||
| − | |||
| − | |||
| − | |||
| − | |||
<br/><br/> | <br/><br/> | ||
| Line 93: | Line 89: | ||
* '''[[SubtInteract|Interactions]]:''' | * '''[[SubtInteract|Interactions]]:''' | ||
| − | + | ** [[YvyD]]-[[ribosome]] (for ribosome dimerization) {{PubMed|22950019}} | |
* '''[[Localization]]:''' | * '''[[Localization]]:''' | ||
** membrane protein | ** membrane protein | ||
| Line 144: | Line 140: | ||
=References= | =References= | ||
| − | <pubmed>11948165,9852014,10913081,22517742,12107147 15805528, </pubmed> | + | <pubmed>11948165,9852014,10913081,22517742,12107147 15805528, 22950019 </pubmed> |
==Publications on the homologous ''E. coli'' proteins Hpf and YfiA== | ==Publications on the homologous ''E. coli'' proteins Hpf and YfiA== | ||
<pubmed> 22605777 </pubmed> | <pubmed> 22605777 </pubmed> | ||
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 09:44, 6 September 2012
- Description: general stress protein, required for ribosome dimerization in the stationary phase
| Gene name | yvyD |
| Synonyms | yviI |
| Essential | no |
| Product | unknown |
| Function | dimerization of ribosomes in the stationary phase |
| Gene expression levels in SubtiExpress: yvyD | |
| MW, pI | 21 kDa, 5.184 |
| Gene length, protein length | 567 bp, 189 aa |
| Immediate neighbours | secA, smiA |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 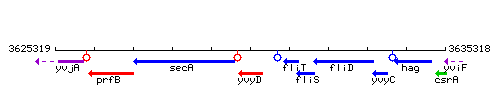
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
general stress proteins (controlled by SigB), membrane proteins, phosphoproteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU35310
Phenotypes of a mutant
- the mutant is cold-sensitive
- delayed recovery from stationary phase and delayed germination
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: ribosomal protein S30Ae family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- phosphorylated on Arg-6 PubMed
- Cofactor(s):
- Effectors of protein activity:
- Interactions:
- Localization:
- membrane protein
Database entries
- Structure:
- UniProt: P28368
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Operon: yvyD PubMed
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Kazumi Tagami, Hideaki Nanamiya, Yuka Kazo, Marie Maehashi, Shota Suzuki, Eri Namba, Masahiro Hoshiya, Ryo Hanai, Yuzuru Tozawa, Takuya Morimoto, Naotake Ogasawara, Yasushi Kageyama, Katsutoshi Ara, Katsuya Ozaki, Masaki Yoshida, Haruko Kuroiwa, Tsuneyoshi Kuroiwa, Yoshiaki Ohashi, Fujio Kawamura
Expression of a small (p)ppGpp synthetase, YwaC, in the (p)ppGpp(0) mutant of Bacillus subtilis triggers YvyD-dependent dimerization of ribosome.
Microbiologyopen: 2012, 1(2);115-34
[PubMed:22950019]
[WorldCat.org]
[DOI]
(I p)
Alexander K W Elsholz, Kürsad Turgay, Stephan Michalik, Bernd Hessling, Katrin Gronau, Dan Oertel, Ulrike Mäder, Jörg Bernhardt, Dörte Becher, Michael Hecker, Ulf Gerth
Global impact of protein arginine phosphorylation on the physiology of Bacillus subtilis.
Proc Natl Acad Sci U S A: 2012, 109(19);7451-6
[PubMed:22517742]
[WorldCat.org]
[DOI]
(I p)
Dirk Höper, Uwe Völker, Michael Hecker
Comprehensive characterization of the contribution of individual SigB-dependent general stress genes to stress resistance of Bacillus subtilis.
J Bacteriol: 2005, 187(8);2810-26
[PubMed:15805528]
[WorldCat.org]
[DOI]
(P p)
Ulrike Mäder, Georg Homuth, Christian Scharf, Knut Büttner, Rüdiger Bode, Michael Hecker
Transcriptome and proteome analysis of Bacillus subtilis gene expression modulated by amino acid availability.
J Bacteriol: 2002, 184(15);4288-95
[PubMed:12107147]
[WorldCat.org]
[DOI]
(P p)
Christine Eymann, Georg Homuth, Christian Scharf, Michael Hecker
Bacillus subtilis functional genomics: global characterization of the stringent response by proteome and transcriptome analysis.
J Bacteriol: 2002, 184(9);2500-20
[PubMed:11948165]
[WorldCat.org]
[DOI]
(P p)
H Antelmann, C Scharf, M Hecker
Phosphate starvation-inducible proteins of Bacillus subtilis: proteomics and transcriptional analysis.
J Bacteriol: 2000, 182(16);4478-90
[PubMed:10913081]
[WorldCat.org]
[DOI]
(P p)
K Drzewiecki, C Eymann, G Mittenhuber, M Hecker
The yvyD gene of Bacillus subtilis is under dual control of sigmaB and sigmaH.
J Bacteriol: 1998, 180(24);6674-80
[PubMed:9852014]
[WorldCat.org]
[DOI]
(P p)
Publications on the homologous E. coli proteins Hpf and YfiA
Yury S Polikanov, Gregor M Blaha, Thomas A Steitz
How hibernation factors RMF, HPF, and YfiA turn off protein synthesis.
Science: 2012, 336(6083);915-8
[PubMed:22605777]
[WorldCat.org]
[DOI]
(I p)