Difference between revisions of "YkuP"
| Line 83: | Line 83: | ||
* '''Modification:''' | * '''Modification:''' | ||
| − | * ''' | + | * '''[[Cofactors]]:''' FMN |
* '''Effectors of protein activity:''' | * '''Effectors of protein activity:''' | ||
| Line 118: | Line 118: | ||
** induced upon iron starvation ([[Fur]]) {{PubMed|12354229}} | ** induced upon iron starvation ([[Fur]]) {{PubMed|12354229}} | ||
** repressed by casamino acids [http://www.ncbi.nlm.nih.gov/pubmed/12107147 PubMed] | ** repressed by casamino acids [http://www.ncbi.nlm.nih.gov/pubmed/12107147 PubMed] | ||
| + | ** expressed under anaerobic conditions ([[ResD]]) {{PubMed|24214949}} | ||
| + | ** induced by nitric oxide under anaerobic conditions ([[NsrR]]) {{PubMed|24214949}} | ||
* '''Regulatory mechanism:''' | * '''Regulatory mechanism:''' | ||
** [[Fur]]: transcription repression {{PubMed|12354229}} | ** [[Fur]]: transcription repression {{PubMed|12354229}} | ||
| + | ** [[ResD]]: transcription activation {{PubMed|24214949}} | ||
| + | ** [[NsrR]]: transcription repression {{PubMed|24214949}} | ||
* '''Additional information:''' | * '''Additional information:''' | ||
| Line 145: | Line 149: | ||
=References= | =References= | ||
| − | + | <pubmed>15449930,17127770 ,12354229,12107147, 20186410 24214949 21665975</pubmed> | |
| − | <pubmed>15449930,17127770 ,12354229,12107147, 20186410</pubmed> | ||
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 15:33, 26 May 2014
- Description: flavodoxin, binds FMN, replaces ferredoxin under conditions of iron limitation
| Gene name | ykuP |
| Synonyms | |
| Essential | no |
| Product | flavodoxin |
| Function | electron transfer |
| Gene expression levels in SubtiExpress: ykuP | |
| Interactions involving this protein in SubtInteract: YkuP | |
| Metabolic function and regulation of this protein in SubtiPathways: ykuP | |
| MW, pI | 20 kDa, 4.098 |
| Gene length, protein length | 534 bp, 178 aa |
| Immediate neighbours | ykuO, ykuQ |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed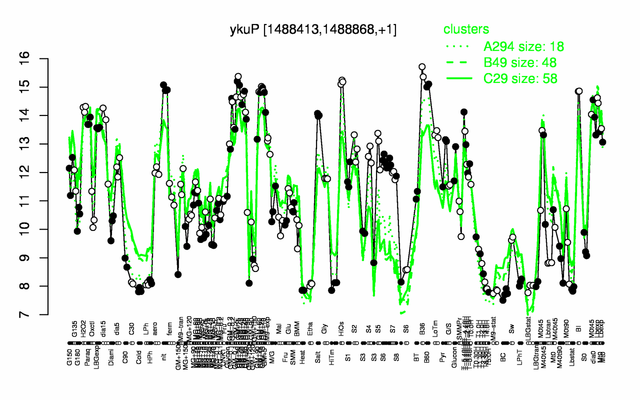
| |
Contents
Categories containing this gene/protein
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU14170
Phenotypes of a mutant
Database entries
- BsubCyc: BSU14170
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: flavodoxin-like domain (according to Swiss-Prot)
- Paralogous protein(s): YkuN
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactors: FMN
- Effectors of protein activity:
Database entries
- BsubCyc: BSU14170
- Structure:
- UniProt: O34589
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Bernadette Henares, Sushma Kommineni, Onuma Chumsakul, Naotake Ogasawara, Shu Ishikawa, Michiko M Nakano
The ResD response regulator, through functional interaction with NsrR and fur, plays three distinct roles in Bacillus subtilis transcriptional control.
J Bacteriol: 2014, 196(2);493-503
[PubMed:24214949]
[WorldCat.org]
[DOI]
(I p)
Lorena Chazarreta-Cifre, Leticia Martiarena, Diego de Mendoza, Silvia G Altabe
Role of ferredoxin and flavodoxins in Bacillus subtilis fatty acid desaturation.
J Bacteriol: 2011, 193(16);4043-8
[PubMed:21665975]
[WorldCat.org]
[DOI]
(I p)
Marco Girhard, Tobias Klaus, Yogan Khatri, Rita Bernhardt, Vlada B Urlacher
Characterization of the versatile monooxygenase CYP109B1 from Bacillus subtilis.
Appl Microbiol Biotechnol: 2010, 87(2);595-607
[PubMed:20186410]
[WorldCat.org]
[DOI]
(I p)
Zhi-Qiang Wang, Rachel J Lawson, Madhavan R Buddha, Chin-Chuan Wei, Brian R Crane, Andrew W Munro, Dennis J Stuehr
Bacterial flavodoxins support nitric oxide production by Bacillus subtilis nitric-oxide synthase.
J Biol Chem: 2007, 282(4);2196-202
[PubMed:17127770]
[WorldCat.org]
[DOI]
(P p)
Rachel J Lawson, Claes von Wachenfeldt, Ihtshamul Haq, John Perkins, Andrew W Munro
Expression and characterization of the two flavodoxin proteins of Bacillus subtilis, YkuN and YkuP: biophysical properties and interactions with cytochrome P450 BioI.
Biochemistry: 2004, 43(39);12390-409
[PubMed:15449930]
[WorldCat.org]
[DOI]
(P p)
Noel Baichoo, Tao Wang, Rick Ye, John D Helmann
Global analysis of the Bacillus subtilis Fur regulon and the iron starvation stimulon.
Mol Microbiol: 2002, 45(6);1613-29
[PubMed:12354229]
[WorldCat.org]
[DOI]
(P p)
Ulrike Mäder, Georg Homuth, Christian Scharf, Knut Büttner, Rüdiger Bode, Michael Hecker
Transcriptome and proteome analysis of Bacillus subtilis gene expression modulated by amino acid availability.
J Bacteriol: 2002, 184(15);4288-95
[PubMed:12107147]
[WorldCat.org]
[DOI]
(P p)