Difference between revisions of "YjbI"
(→References) |
|||
| Line 136: | Line 136: | ||
=References= | =References= | ||
| − | <pubmed>17293416,19899166 , 20102180 </pubmed> | + | <pubmed>17293416,19899166 , 20102180 22759230 </pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 16:41, 6 July 2012
- Description: truncated hemoglobin, NO protection
| Gene name | yjbI |
| Synonyms | |
| Essential | no |
| Product | truncated hemoglobin |
| Function | protection against NO, thiol redox homeostasis |
| MW, pI | 14 kDa, 5.173 |
| Gene length, protein length | 396 bp, 132 aa |
| Immediate neighbours | yjbH, cwlQ |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 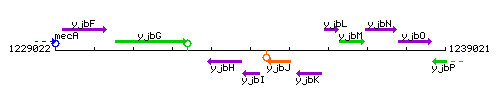
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
resistance against other toxic compounds (nitric oxide, phenolic acids, flavonoids, oxalate)
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU11560
Phenotypes of a mutant
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: binds sulfide with high affinity PubMed
- Protein family: protozoan/cyanobacterial globin family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information: K(D) for hydrogen sulfide = 5.0x10(6) M-1 PubMed
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Interactions:
- Localization:
Database entries
- Structure: 1UX8
- UniProt: O31607
- KEGG entry: [2]
- E.C. number:
Additional information
Expression and regulation
- Sigma factor:
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Andrea Lapini, Mariangela Di Donato, Barbara Patrizi, Agnese Marcelli, Manuela Lima, Roberto Righini, Paolo Foggi, Natascia Sciamanna, Alberto Boffi
Carbon monoxide recombination dynamics in truncated hemoglobins studied with visible-pump midIR-probe spectroscopy.
J Phys Chem B: 2012, 116(30);8753-61
[PubMed:22759230]
[WorldCat.org]
[DOI]
(I p)
Francesco P Nicoletti, Alessandra Comandini, Alessandra Bonamore, Leonardo Boechi, Fernando Martin Boubeta, Alessandro Feis, Giulietta Smulevich, Alberto Boffi
Sulfide binding properties of truncated hemoglobins.
Biochemistry: 2010, 49(10);2269-78
[PubMed:20102180]
[WorldCat.org]
[DOI]
(I p)
Leonardo Boechi, Pau Arroyo Mañez, F Javier Luque, Marcelo A Marti, Dario A Estrin
Unraveling the molecular basis for ligand binding in truncated hemoglobins: the trHbO Bacillus subtilis case.
Proteins: 2010, 78(4);962-70
[PubMed:19899166]
[WorldCat.org]
[DOI]
(I p)
Annika Rogstam, Jonas T Larsson, Peter Kjelgaard, Claes von Wachenfeldt
Mechanisms of adaptation to nitrosative stress in Bacillus subtilis.
J Bacteriol: 2007, 189(8);3063-71
[PubMed:17293416]
[WorldCat.org]
[DOI]
(P p)