Difference between revisions of "YhfR"
| Line 26: | Line 26: | ||
<div align="right"> <small>This image was kindly provided by [http://genolist.pasteur.fr/SubtiList/ SubtiList]</small></div> | <div align="right"> <small>This image was kindly provided by [http://genolist.pasteur.fr/SubtiList/ SubtiList]</small></div> | ||
|- | |- | ||
| − | |colspan="2" |'''[http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=phoE_1108736_1109317_-1 Expression at a glance]'''   {{PubMed|22383849}}<br/>[[Image:yhfR_expression.png|500px]] | + | |colspan="2" |'''[http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=phoE_1108736_1109317_-1 Expression at a glance]'''   {{PubMed|22383849}}<br/>[[Image:yhfR_expression.png|500px|link=http://subtiwiki.uni-goettingen.de/apps/expression/expression.php?search=BSU10340]] |
|- | |- | ||
|} | |} | ||
Revision as of 12:54, 16 May 2013
- Description: phosphatase involved in isopentenol (isoprenoid) biosynthesis
| Gene name | yhfR |
| Synonyms | |
| Essential | no |
| Product | unknown |
| Function | isoprenoid biosynthesis |
| Gene expression levels in SubtiExpress: yhfR | |
| MW, pI | 21 kDa, 5.164 |
| Gene length, protein length | 579 bp, 193 aa |
| Immediate neighbours | yhfQ, yhfS |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 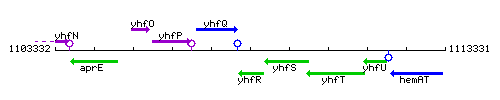
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed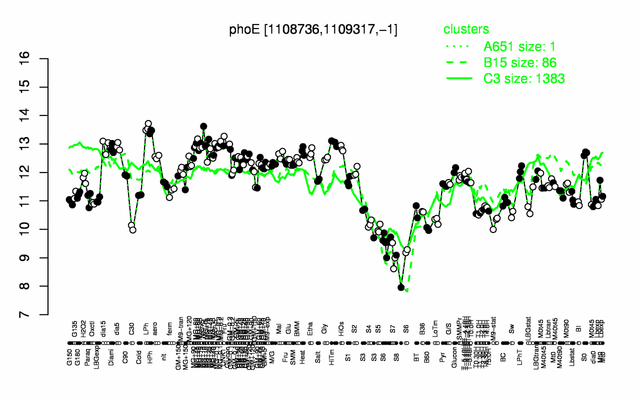
| |
Contents
Categories containing this gene/protein
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU10340
Phenotypes of a mutant
no detectable phenotype PubMed
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: phosphatase involved in isopentenol (isoprenoid) biosynthesis
- Protein family: GpmB subfamily (according to Swiss-Prot) similar to 2,3-diphosphoglycerate-dependent phosphoglycerate mutases PubMed
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- UniProt: O07617
- KEGG entry: [2]
- E.C. number:
Additional information
The gene is annotated in KEGG as an ortholog of phosphoglycerate mutase (PGM) EC 5.4.2.1. In MetaCyc the protein is marked as “similar to phosphoglycerate mutase”. No EC annotation is available in Swiss-Prot. Pearson et al. (PubMed) demonstrated that yhfR is non-essential for growth, sporulation, and spore germination. They also purified the gene, expressed it in B. subtilis but were not able to detect PGM activity in B. subtilis. PubMed
Expression and regulation
- Operon:
- Sigma factor:
- Regulation:
- Regulatory mechanism:
- Additional information: weakly expressed PubMed
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Tzu-Lin Hsiao, Olga Revelles, Lifeng Chen, Uwe Sauer, Dennis Vitkup
Automatic policing of biochemical annotations using genomic correlations.
Nat Chem Biol: 2010, 6(1);34-40
[PubMed:19935659]
[WorldCat.org]
[DOI]
(I p)
Sydnor T Withers, Shayin S Gottlieb, Bonny Lieu, Jack D Newman, Jay D Keasling
Identification of isopentenol biosynthetic genes from Bacillus subtilis by a screening method based on isoprenoid precursor toxicity.
Appl Environ Microbiol: 2007, 73(19);6277-83
[PubMed:17693564]
[WorldCat.org]
[DOI]
(P p)
Daniel J Rigden, Luciane V Mello, Peter Setlow, Mark J Jedrzejas
Structure and mechanism of action of a cofactor-dependent phosphoglycerate mutase homolog from Bacillus stearothermophilus with broad specificity phosphatase activity.
J Mol Biol: 2002, 315(5);1129-43
[PubMed:11827481]
[WorldCat.org]
[DOI]
(P p)
D J Rigden, I Bagyan, E Lamani, P Setlow, M J Jedrzejas
A cofactor-dependent phosphoglycerate mutase homolog from Bacillus stearothermophilus is actually a broad specificity phosphatase.
Protein Sci: 2001, 10(9);1835-46
[PubMed:11514674]
[WorldCat.org]
[DOI]
(P p)
C L Pearson, C A Loshon, L B Pedersen, B Setlow, P Setlow
Analysis of the function of a putative 2,3-diphosphoglyceric acid-dependent phosphoglycerate mutase from Bacillus subtilis.
J Bacteriol: 2000, 182(14);4121-3
[PubMed:10869096]
[WorldCat.org]
[DOI]
(P p)