Difference between revisions of "Vpr"
| Line 42: | Line 42: | ||
= This gene is a member of the following [[regulons]] = | = This gene is a member of the following [[regulons]] = | ||
| + | {{SubtiWiki regulon|[[CodY regulon]]}}, | ||
{{SubtiWiki regulon|[[LexA regulon]]}}, | {{SubtiWiki regulon|[[LexA regulon]]}}, | ||
{{SubtiWiki regulon|[[PhoP regulon]]}} | {{SubtiWiki regulon|[[PhoP regulon]]}} | ||
| Line 113: | Line 114: | ||
** expressed under conditions of phosphate limitation ([[PhoP]]) [http://www.ncbi.nlm.nih.gov/sites/entrez/16291680 PubMed] | ** expressed under conditions of phosphate limitation ([[PhoP]]) [http://www.ncbi.nlm.nih.gov/sites/entrez/16291680 PubMed] | ||
** repressed when no DNA damage is present ([[LexA]]) [http://www.ncbi.nlm.nih.gov/sites/entrez/16267290 PubMed] | ** repressed when no DNA damage is present ([[LexA]]) [http://www.ncbi.nlm.nih.gov/sites/entrez/16267290 PubMed] | ||
| + | ** repressed during growth in the presence of branched chain amino acids ([[CodY]]) {{PubMed|25666135}} | ||
* '''Regulatory mechanism:''' | * '''Regulatory mechanism:''' | ||
| + | ** [[CodY]]: transcription repression {{PubMed|25666135}} | ||
** [[PhoP]]: transcription activation [http://www.ncbi.nlm.nih.gov/sites/entrez/16291680 PubMed] | ** [[PhoP]]: transcription activation [http://www.ncbi.nlm.nih.gov/sites/entrez/16291680 PubMed] | ||
** [[LexA]]: transcription repression [http://www.ncbi.nlm.nih.gov/sites/entrez/16267290 PubMed] | ** [[LexA]]: transcription repression [http://www.ncbi.nlm.nih.gov/sites/entrez/16267290 PubMed] | ||
| Line 144: | Line 147: | ||
<pubmed>20735481 </pubmed> | <pubmed>20735481 </pubmed> | ||
==Original publications== | ==Original publications== | ||
| − | <pubmed>2106512, 24115457,16291680,16267290,18957862, </pubmed> | + | <pubmed>2106512, 24115457,16291680,16267290,18957862, 25666135</pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 12:26, 11 February 2015
- Description: minor extracellular serine protease
| Gene name | vpr |
| Synonyms | ipa-45r |
| Essential | no |
| Product | minor extracellular serine protease |
| Function | protein degradation |
| Gene expression levels in SubtiExpress: vpr | |
| MW, pI | 85 kDa, 5.773 |
| Gene length, protein length | 2418 bp, 806 aa |
| Immediate neighbours | ywcI, ywcH |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 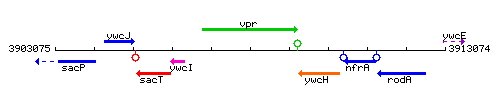
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed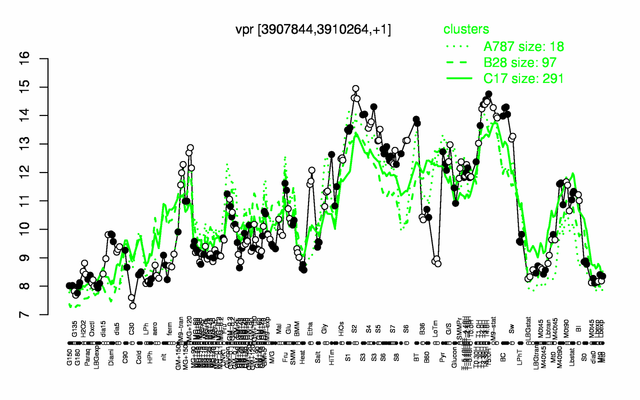
| |
Contents
Categories containing this gene/protein
utilization of nitrogen sources other than amino acids, proteolysis
This gene is a member of the following regulons
CodY regulon, LexA regulon, PhoP regulon
The gene
Basic information
- Locus tag: BSU38090
Phenotypes of a mutant
Database entries
- BsubCyc: BSU38090
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: peptidase S8 family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Modification:
- Effectors of protein activity:
- Localization:
- extracellular (signal peptide) PubMed
Database entries
- BsubCyc: BSU38090
- Structure:
- UniProt: P29141
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Operon: vpr PubMed
- Regulation:
- Regulatory mechanism:
- Additional information:
- number of protein molecules per cell (minimal medium with glucose and ammonium, exponential phase): 248 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium, early stationary phase after glucose exhaustion): 175 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium, late stationary phase after glucose exhaustion): 224 PubMed
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Massimiliano Marvasi, Pieter T Visscher, Lilliam Casillas Martinez
Exopolymeric substances (EPS) from Bacillus subtilis: polymers and genes encoding their synthesis.
FEMS Microbiol Lett: 2010, 313(1);1-9
[PubMed:20735481]
[WorldCat.org]
[DOI]
(I p)
Original publications
Giulia Barbieri, Birgit Voigt, Dirk Albrecht, Michael Hecker, Alessandra M Albertini, Abraham L Sonenshein, Eugenio Ferrari, Boris R Belitsky
CodY regulates expression of the Bacillus subtilis extracellular proteases Vpr and Mpr.
J Bacteriol: 2015, 197(8);1423-32
[PubMed:25666135]
[WorldCat.org]
[DOI]
(I p)
Susanne Pohl, Gaurav Bhavsar, Joanne Hulme, Alexandra E Bloor, Goksel Misirli, Matthew W Leckenby, David S Radford, Wendy Smith, Anil Wipat, E Diane Williamson, Colin R Harwood, Rocky M Cranenburgh
Proteomic analysis of Bacillus subtilis strains engineered for improved production of heterologous proteins.
Proteomics: 2013, 13(22);3298-308
[PubMed:24115457]
[WorldCat.org]
[DOI]
(I p)
Birgit Voigt, Haike Antelmann, Dirk Albrecht, Armin Ehrenreich, Karl-Heinz Maurer, Stefan Evers, Gerhard Gottschalk, Jan Maarten van Dijl, Thomas Schweder, Michael Hecker
Cell physiology and protein secretion of Bacillus licheniformis compared to Bacillus subtilis.
J Mol Microbiol Biotechnol: 2009, 16(1-2);53-68
[PubMed:18957862]
[WorldCat.org]
[DOI]
(I p)
Nicholas E E Allenby, Nicola O'Connor, Zoltán Prágai, Alan C Ward, Anil Wipat, Colin R Harwood
Genome-wide transcriptional analysis of the phosphate starvation stimulon of Bacillus subtilis.
J Bacteriol: 2005, 187(23);8063-80
[PubMed:16291680]
[WorldCat.org]
[DOI]
(P p)
Nora Au, Elke Kuester-Schoeck, Veena Mandava, Laura E Bothwell, Susan P Canny, Karen Chachu, Sierra A Colavito, Shakierah N Fuller, Eli S Groban, Laura A Hensley, Theresa C O'Brien, Amish Shah, Jessica T Tierney, Louise L Tomm, Thomas M O'Gara, Alexi I Goranov, Alan D Grossman, Charles M Lovett
Genetic composition of the Bacillus subtilis SOS system.
J Bacteriol: 2005, 187(22);7655-66
[PubMed:16267290]
[WorldCat.org]
[DOI]
(P p)
A Sloma, G A Rufo, C F Rudolph, B J Sullivan, K A Theriault, J Pero
Bacillopeptidase F of Bacillus subtilis: purification of the protein and cloning of the gene.
J Bacteriol: 1990, 172(3);1470-7
[PubMed:2106512]
[WorldCat.org]
[DOI]
(P p)