Difference between revisions of "TsaD"
Raphael2215 (talk | contribs) |
(→Categories containing this gene/protein) |
||
| Line 45: | Line 45: | ||
{{SubtiWiki category|[[translation]]}}, | {{SubtiWiki category|[[translation]]}}, | ||
{{SubtiWiki category|[[essential genes]]}}, | {{SubtiWiki category|[[essential genes]]}}, | ||
| − | {{SubtiWiki category|[[universally conserved proteins | + | {{SubtiWiki category|[[universally conserved proteins]]}} |
| − | |||
= This gene is a member of the following [[regulons]] = | = This gene is a member of the following [[regulons]] = | ||
Revision as of 16:12, 28 August 2012
- Description: there are two annotations for the corresponding E. coli protein: O-sialoglycoprotein endopeptidase, and required for threonyl carbamoyl adenosine (t6A) modification of tRNAs that pair with ANN codons in mRNA (together with YwlC), universally conserved protein
| Gene name | gcp |
| Synonyms | ydiE |
| Essential | yes PubMed |
| Product | O-sialoglycoprotein endopeptidase (putative) |
| Function | tRNA modification |
| Gene expression levels in SubtiExpress: gcp | |
| Interactions involving this protein in SubtInteract: Gcp | |
| MW, pI | 36 kDa, 5.016 |
| Gene length, protein length | 1038 bp, 346 aa |
| Immediate neighbours | ydiD, ydiF |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 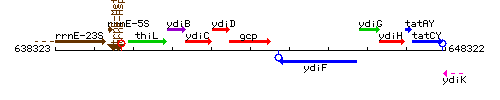
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
translation, essential genes, universally conserved proteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU05940
Phenotypes of a mutant
essential PubMed
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: peptidase M22 family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- Structure:
- UniProt: O05518
- KEGG entry: [2]
- E.C. number:
Additional information
Expression and regulation
- Operon:
- Sigma factor:
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Publications on the corresponding E. coli protein, YgjD
Tobias Bergmiller, Rafael Peña-Miller, Alexander Boehm, Martin Ackermann
Single-cell time-lapse analysis of depletion of the universally conserved essential protein YgjD.
BMC Microbiol: 2011, 11;118
[PubMed:21619589]
[WorldCat.org]
[DOI]
(I e)
Basma El Yacoubi, Isabelle Hatin, Christopher Deutsch, Tamer Kahveci, Jean-Pierre Rousset, Dirk Iwata-Reuyl, Alexey G Murzin, Valérie de Crécy-Lagard
A role for the universal Kae1/Qri7/YgjD (COG0533) family in tRNA modification.
EMBO J: 2011, 30(5);882-93
[PubMed:21285948]
[WorldCat.org]
[DOI]
(I p)
Madhusudhan Srinivasan, Preeti Mehta, Yao Yu, Evelyn Prugar, Eugene V Koonin, A Wali Karzai, Rolf Sternglanz
The highly conserved KEOPS/EKC complex is essential for a universal tRNA modification, t6A.
EMBO J: 2011, 30(5);873-81
[PubMed:21183954]
[WorldCat.org]
[DOI]
(I p)
Chen Katz, Ifat Cohen-Or, Uri Gophna, Eliora Z Ron
The ubiquitous conserved glycopeptidase Gcp prevents accumulation of toxic glycated proteins.
mBio: 2010, 1(3);
[PubMed:20824107]
[WorldCat.org]
[DOI]
(I e)
Jennifer I Handford, Bérengère Ize, Grant Buchanan, Gareth P Butland, Jack Greenblatt, Andrew Emili, Tracy Palmer
Conserved network of proteins essential for bacterial viability.
J Bacteriol: 2009, 191(15);4732-49
[PubMed:19376873]
[WorldCat.org]
[DOI]
(I p)