Difference between revisions of "TrxA"
(→References) |
|||
| Line 74: | Line 74: | ||
* '''Effectors of protein activity:''' | * '''Effectors of protein activity:''' | ||
| − | * '''Interactions:''' [[ | + | * '''Interactions:''' [[ArsC]]-[[TrxA]] {{PubMed|17303556}} |
* '''Localization:''' cytoplasm (according to Swiss-Prot) | * '''Localization:''' cytoplasm (according to Swiss-Prot) | ||
| Line 80: | Line 80: | ||
=== Database entries === | === Database entries === | ||
| − | * '''Structure:''' [http://www.rcsb.org/pdb/explore.do?structureId=2GZZ 2GZZ] (oxidized form), [http://www.rcsb.org/pdb/explore.do?structureId=2GZY 2GZY] (reduced form), [http://www.rcsb.org/pdb/explore.do?structureId=2IPA 2IPA] (TrxA-ArsC-complex) | + | * '''Structure:''' [http://www.rcsb.org/pdb/explore.do?structureId=2GZZ 2GZZ] (oxidized form), [http://www.rcsb.org/pdb/explore.do?structureId=2GZY 2GZY] (reduced form), [http://www.rcsb.org/pdb/explore.do?structureId=2IPA 2IPA] (TrxA-ArsC-complex) {{PubMed|17303556}} |
* '''UniProt:''' [http://www.uniprot.org/uniprot/P14949 P14949] | * '''UniProt:''' [http://www.uniprot.org/uniprot/P14949 P14949] | ||
| Line 126: | Line 126: | ||
=References= | =References= | ||
| − | <pubmed>9537387,15937154,18601268,18456801,9852015 ,11544224,18687074 20084284 </pubmed> | + | <pubmed>9537387,15937154,18601268,18456801,9852015 ,11544224,18687074 20084284 17303556</pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 06:40, 24 February 2010
- Description: antioxidative action by facilitating the reduction of other proteins by cysteine thiol-disulfide exchange
| Gene name | trxA |
| Synonyms | trx |
| Essential | yes PubMed |
| Product | thioredoxin |
| Function | protection of proteins against oxidative damage |
| MW, pI | 11 kDa, 4.308 |
| Gene length, protein length | 312 bp, 104 aa |
| Immediate neighbours | uvrC, abf2 |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 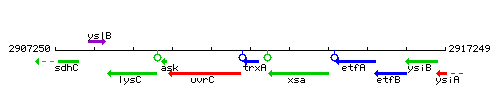
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU28500
Phenotypes of a mutant
essential PubMed
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: thioredoxin family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization: cytoplasm (according to Swiss-Prot)
Database entries
- UniProt: P14949
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Operon: trxA PubMed
- Regulation:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Michiko M Nakano, Ann Lin, Cole S Zuber, Kate J Newberry, Richard G Brennan, Peter Zuber
Promoter recognition by a complex of Spx and the C-terminal domain of the RNA polymerase alpha subunit.
PLoS One: 2010, 5(1);e8664
[PubMed:20084284]
[WorldCat.org]
[DOI]
(I e)
Dindo Y Reyes, Peter Zuber
Activation of transcription initiation by Spx: formation of transcription complex and identification of a Cis-acting element required for transcriptional activation.
Mol Microbiol: 2008, 69(3);765-79
[PubMed:18687074]
[WorldCat.org]
[DOI]
(I p)
Jörg Mostertz, Falko Hochgräfe, Britta Jürgen, Thomas Schweder, Michael Hecker
The role of thioredoxin TrxA in Bacillus subtilis: a proteomics and transcriptomics approach.
Proteomics: 2008, 8(13);2676-90
[PubMed:18601268]
[WorldCat.org]
[DOI]
(I p)
Mirja Carlsson Möller, Lars Hederstedt
Extracytoplasmic processes impaired by inactivation of trxA (thioredoxin gene) in Bacillus subtilis.
J Bacteriol: 2008, 190(13);4660-5
[PubMed:18456801]
[WorldCat.org]
[DOI]
(I p)
You Li, Yunfei Hu, Xinxin Zhang, Huimin Xu, Ewen Lescop, Bin Xia, Changwen Jin
Conformational fluctuations coupled to the thiol-disulfide transfer between thioredoxin and arsenate reductase in Bacillus subtilis.
J Biol Chem: 2007, 282(15);11078-83
[PubMed:17303556]
[WorldCat.org]
[DOI]
(P p)
Wiep Klaas Smits, Jean-Yves F Dubois, Sierd Bron, Jan Maarten van Dijl, Oscar P Kuipers
Tricksy business: transcriptome analysis reveals the involvement of thioredoxin A in redox homeostasis, oxidative stress, sulfur metabolism, and cellular differentiation in Bacillus subtilis.
J Bacteriol: 2005, 187(12);3921-30
[PubMed:15937154]
[WorldCat.org]
[DOI]
(P p)
A Petersohn, M Brigulla, S Haas, J D Hoheisel, U Völker, M Hecker
Global analysis of the general stress response of Bacillus subtilis.
J Bacteriol: 2001, 183(19);5617-31
[PubMed:11544224]
[WorldCat.org]
[DOI]
(P p)
E Krüger, M Hecker
The first gene of the Bacillus subtilis clpC operon, ctsR, encodes a negative regulator of its own operon and other class III heat shock genes.
J Bacteriol: 1998, 180(24);6681-8
[PubMed:9852015]
[WorldCat.org]
[DOI]
(P p)
C Scharf, S Riethdorf, H Ernst, S Engelmann, U Völker, M Hecker
Thioredoxin is an essential protein induced by multiple stresses in Bacillus subtilis.
J Bacteriol: 1998, 180(7);1869-77
[PubMed:9537387]
[WorldCat.org]
[DOI]
(P p)