Difference between revisions of "TilS"
(→Original publications) |
|||
| Line 150: | Line 150: | ||
<pubmed>15124629 ,19944692 </pubmed> | <pubmed>15124629 ,19944692 </pubmed> | ||
==Original publications== | ==Original publications== | ||
| − | <pubmed>,14527414, 16479537, 17005971, 19233850 19847269 21435031 18179421 24001521 </pubmed> | + | <pubmed>,14527414, 16479537, 17005971, 19233850 19847269 21435031 18179421 24001521 24194599</pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 17:40, 17 November 2013
- Description: trigger enzyme, tRNAIle-lysidine synthetase and part of a transcription activator
| Gene name | tilS |
| Synonyms | yacA |
| Essential | yes PubMed |
| Product | tRNAIle-lysidine synthetase |
| Function | tRNA modification, control of ftsH expression |
| Gene expression levels in SubtiExpress: tilS | |
| Interactions involving this protein in SubtInteract: TilS | |
| Metabolic function and regulation of this protein in SubtiPathways: Nucleotides (regulation) | |
| MW, pI | 53 kDa, 8.317 |
| Gene length, protein length | 1416 bp, 472 aa |
| Immediate neighbours | yabT, hprT |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 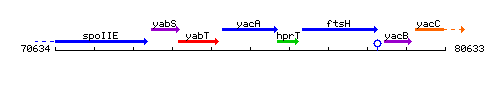
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed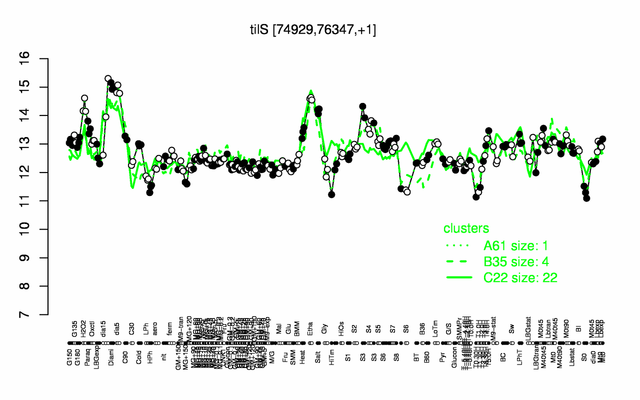
| |
Contents
Categories containing this gene/protein
translation, transcription factors and their control, cell envelope stress proteins (controlled by SigM, V, W, X, Y), essential genes
This gene is a member of the following regulons
The TilS-HprT regulon
The gene
Basic information
- Locus tag: BSU00670
Phenotypes of a mutant
essential PubMed
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: tRNA(Ile)-lysidine synthase family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization:
- Cytoplasm (Homogeneous) PubMed
Database entries
- UniProt: P37563
- KEGG entry: [2]
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Tsutomu Suzuki, Kenjyo Miyauchi
Discovery and characterization of tRNAIle lysidine synthetase (TilS).
FEBS Lett: 2010, 584(2);272-7
[PubMed:19944692]
[WorldCat.org]
[DOI]
(I p)
Henri Grosjean, Glenn R Björk
Enzymatic conversion of cytidine to lysidine in anticodon of bacterial isoleucyl-tRNA--an alternative way of RNA editing.
Trends Biochem Sci: 2004, 29(4);165-8
[PubMed:15124629]
[WorldCat.org]
[DOI]
(P p)
Original publications
Caroline Köhrer, Debabrata Mandal, Kirk W Gaston, Henri Grosjean, Patrick A Limbach, Uttam L Rajbhandary
Life without tRNAIle-lysidine synthetase: translation of the isoleucine codon AUA in Bacillus subtilis lacking the canonical tRNA2Ile.
Nucleic Acids Res: 2014, 42(3);1904-15
[PubMed:24194599]
[WorldCat.org]
[DOI]
(I p)
Ta-Hui Lin, Yi-Nei Hu, Gwo-Chyuan Shaw
Two enzymes, TilS and HprT, can form a complex to function as a transcriptional activator for the cell division protease gene ftsH in Bacillus subtilis.
J Biochem: 2014, 155(1);5-16
[PubMed:24001521]
[WorldCat.org]
[DOI]
(I p)
Céline Fabret, Etienne Dervyn, Bérengère Dalmais, Alain Guillot, Christian Marck, Henri Grosjean, Philippe Noirot
Life without the essential bacterial tRNA Ile2-lysidine synthetase TilS: a case of tRNA gene recruitment in Bacillus subtilis.
Mol Microbiol: 2011, 80(4);1062-74
[PubMed:21435031]
[WorldCat.org]
[DOI]
(I p)
Kotaro Nakanishi, Luc Bonnefond, Satoshi Kimura, Tsutomu Suzuki, Ryuichiro Ishitani, Osamu Nureki
Structural basis for translational fidelity ensured by transfer RNA lysidine synthetase.
Nature: 2009, 461(7267);1144-8
[PubMed:19847269]
[WorldCat.org]
[DOI]
(I p)
Scott P Salowe, Judyann Wiltsie, Julio C Hawkins, Lisa M Sonatore
The catalytic flexibility of tRNAIle-lysidine synthetase can generate alternative tRNA substrates for isoleucyl-tRNA synthetase.
J Biol Chem: 2009, 284(15);9656-62
[PubMed:19233850]
[WorldCat.org]
[DOI]
(P p)
Warawan Eiamphungporn, John D Helmann
The Bacillus subtilis sigma(M) regulon and its contribution to cell envelope stress responses.
Mol Microbiol: 2008, 67(4);830-48
[PubMed:18179421]
[WorldCat.org]
[DOI]
(P p)
Alison Hunt, Joy P Rawlins, Helena B Thomaides, Jeff Errington
Functional analysis of 11 putative essential genes in Bacillus subtilis.
Microbiology (Reading): 2006, 152(Pt 10);2895-2907
[PubMed:17005971]
[WorldCat.org]
[DOI]
(P p)
Jean-Christophe Meile, Ling Juan Wu, S Dusko Ehrlich, Jeff Errington, Philippe Noirot
Systematic localisation of proteins fused to the green fluorescent protein in Bacillus subtilis: identification of new proteins at the DNA replication factory.
Proteomics: 2006, 6(7);2135-46
[PubMed:16479537]
[WorldCat.org]
[DOI]
(P p)
Akiko Soma, Yoshiho Ikeuchi, Satoru Kanemasa, Kazuo Kobayashi, Naotake Ogasawara, Tomotake Ote, Jun-ichi Kato, Kimitsuna Watanabe, Yasuhiko Sekine, Tsutomu Suzuki
An RNA-modifying enzyme that governs both the codon and amino acid specificities of isoleucine tRNA.
Mol Cell: 2003, 12(3);689-98
[PubMed:14527414]
[WorldCat.org]
[DOI]
(P p)