Difference between revisions of "Tig"
| Line 14: | Line 14: | ||
|style="background:#ABCDEF;" align="center"|'''Function''' || protein folding | |style="background:#ABCDEF;" align="center"|'''Function''' || protein folding | ||
|- | |- | ||
| − | |colspan="2" style="background:#FAF8CC;" align="center"| '''Gene expression levels in [http:// | + | |colspan="2" style="background:#FAF8CC;" align="center"| '''Gene expression levels in [http://subtiwiki.uni-goettingen.de/apps/expression/ ''Subti''Express]''': [http://subtiwiki.uni-goettingen.de/apps/expression/expression.php?search=BSU28230 tig] |
|- | |- | ||
|style="background:#ABCDEF;" align="center"| '''MW, pI''' || 47 kDa, 4.224 | |style="background:#ABCDEF;" align="center"| '''MW, pI''' || 47 kDa, 4.224 | ||
| Line 22: | Line 22: | ||
|style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[clpX]]'', ''[[ysoA]]'' | |style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[clpX]]'', ''[[ysoA]]'' | ||
|- | |- | ||
| − | | | + | |style="background:#FAF8CC;" align="center"|'''Sequences'''||[http://bsubcyc.org/BSUB/sequence-aa?type=GENE&object=BSU28230 Protein] [http://bsubcyc.org/BSUB/sequence?type=GENE&object=BSU28230 DNA] [http://bsubcyc.org/BSUB/seq-selector?chromosome=CHROM-1&object=BSU28230 Advanced_DNA] |
|- | |- | ||
|colspan="2" | '''Genetic context''' <br/> [[Image:tig_context.gif]] | |colspan="2" | '''Genetic context''' <br/> [[Image:tig_context.gif]] | ||
Revision as of 13:30, 13 May 2013
- Description: trigger factor (prolyl isomerase)
| Gene name | tig |
| Synonyms | yzzH |
| Essential | no |
| Product | trigger factor (prolyl isomerase) |
| Function | protein folding |
| Gene expression levels in SubtiExpress: tig | |
| MW, pI | 47 kDa, 4.224 |
| Gene length, protein length | 1272 bp, 424 aa |
| Immediate neighbours | clpX, ysoA |
| Sequences | Protein DNA Advanced_DNA |
Genetic context 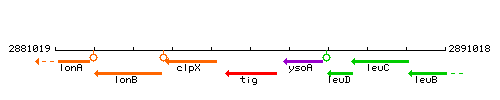
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
chaperones/ protein folding, phosphoproteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU28230
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: Tig subfamily (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- UniProt: P80698
- KEGG entry: [3]
- E.C. number:
Additional information
- subject to Clp-dependent proteolysis upon glucose starvation PubMed
Expression and regulation
- Operon: tig PubMed
- Sigma factor:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Carmela Giglione, Sonia Fieulaine, Thierry Meinnel
Cotranslational processing mechanisms: towards a dynamic 3D model.
Trends Biochem Sci: 2009, 34(8);417-26
[PubMed:19647435]
[WorldCat.org]
[DOI]
(I p)
R D Wegrzyn, E Deuerling
Molecular guardians for newborn proteins: ribosome-associated chaperones and their role in protein folding.
Cell Mol Life Sci: 2005, 62(23);2727-38
[PubMed:16231086]
[WorldCat.org]
[DOI]
(P p)
Timm Maier, Lars Ferbitz, Elke Deuerling, Nenad Ban
A cradle for new proteins: trigger factor at the ribosome.
Curr Opin Struct Biol: 2005, 15(2);204-12
[PubMed:15837180]
[WorldCat.org]
[DOI]
(P p)
Elke Deuerling, Bernd Bukau
Chaperone-assisted folding of newly synthesized proteins in the cytosol.
Crit Rev Biochem Mol Biol: 2004, 39(5-6);261-77
[PubMed:15763705]
[WorldCat.org]
[DOI]
(P p)
Original publications
Alexander K W Elsholz, Kürsad Turgay, Stephan Michalik, Bernd Hessling, Katrin Gronau, Dan Oertel, Ulrike Mäder, Jörg Bernhardt, Dörte Becher, Michael Hecker, Ulf Gerth
Global impact of protein arginine phosphorylation on the physiology of Bacillus subtilis.
Proc Natl Acad Sci U S A: 2012, 109(19);7451-6
[PubMed:22517742]
[WorldCat.org]
[DOI]
(I p)
Irnov Irnov, Cynthia M Sharma, Jörg Vogel, Wade C Winkler
Identification of regulatory RNAs in Bacillus subtilis.
Nucleic Acids Res: 2010, 38(19);6637-51
[PubMed:20525796]
[WorldCat.org]
[DOI]
(I p)
Frieder Merz, Daniel Boehringer, Christiane Schaffitzel, Steffen Preissler, Anja Hoffmann, Timm Maier, Anna Rutkowska, Jasmin Lozza, Nenad Ban, Bernd Bukau, Elke Deuerling
Molecular mechanism and structure of Trigger Factor bound to the translating ribosome.
EMBO J: 2008, 27(11);1622-32
[PubMed:18497744]
[WorldCat.org]
[DOI]
(I p)
Alain Lévine, Françoise Vannier, Cédric Absalon, Lauriane Kuhn, Peter Jackson, Elaine Scrivener, Valérie Labas, Joëlle Vinh, Patrick Courtney, Jérôme Garin, Simone J Séror
Analysis of the dynamic Bacillus subtilis Ser/Thr/Tyr phosphoproteome implicated in a wide variety of cellular processes.
Proteomics: 2006, 6(7);2157-73
[PubMed:16493705]
[WorldCat.org]
[DOI]
(P p)
Dindo Y Reyes, Hirofumi Yoshikawa
DnaK chaperone machine and trigger factor are only partially required for normal growth of Bacillus subtilis.
Biosci Biotechnol Biochem: 2002, 66(7);1583-6
[PubMed:12224648]
[WorldCat.org]
[DOI]
(P p)
S F Göthel, C Scholz, F X Schmid, M A Marahiel
Cyclophilin and trigger factor from Bacillus subtilis catalyze in vitro protein folding and are necessary for viability under starvation conditions.
Biochemistry: 1998, 37(38);13392-9
[PubMed:9748346]
[WorldCat.org]
[DOI]
(P p)
S F Göthel, R Schmid, A Wipat, N M Carter, P T Emmerson, C R Harwood, M A Marahiel
An internal FK506-binding domain is the catalytic core of the prolyl isomerase activity associated with the Bacillus subtilis trigger factor.
Eur J Biochem: 1997, 244(1);59-65
[PubMed:9063446]
[WorldCat.org]
[DOI]
(P p)
A Wipat, N Carter, S C Brignell, B J Guy, K Piper, J Sanders, P T Emmerson, C R Harwood
The dnaB-pheA (256 degrees-240 degrees) region of the Bacillus subtilis chromosome containing genes responsible for stress responses, the utilization of plant cell walls and primary metabolism.
Microbiology (Reading): 1996, 142 ( Pt 11);3067-78
[PubMed:8969504]
[WorldCat.org]
[DOI]
(P p)