ThrB
- Description: homoserine kinase
| Gene name | thrB |
| Synonyms | thrA |
| Essential | no |
| Product | homoserine kinase |
| Function | biosynthesis of threonine |
| Gene expression levels in SubtiExpress: thrB | |
| Metabolic function and regulation of this protein in SubtiPathways: thrB | |
| MW, pI | 33 kDa, 4.735 |
| Gene length, protein length | 927 bp, 309 aa |
| Immediate neighbours | yuxL, thrC |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 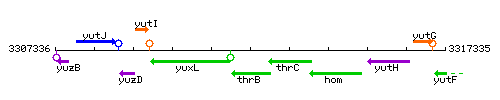
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed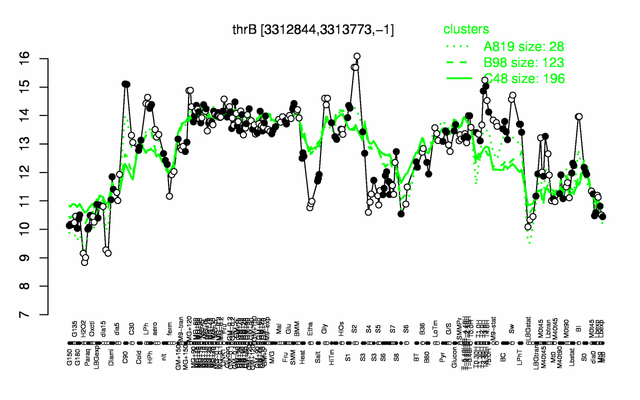
| |
Contents
Categories containing this gene/protein
biosynthesis/ acquisition of amino acids
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU32240
Phenotypes of a mutant
Database entries
- BsubCyc: BSU32240
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: ATP + L-homoserine = ADP + O-phospho-L-homoserine (according to Swiss-Prot)
- Protein family: Homoserine kinase subfamily (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Modification:
- Effectors of protein activity:
- Localization: cytoplasm (according to Swiss-Prot)
Database entries
- BsubCyc: BSU32240
- Structure: 3HUL (from Listeria monocytogenes, 41% identity, 61% similarity)
- UniProt: P04948
- KEGG entry: [2]
- E.C. number: 2.7.1.39
Additional information
Expression and regulation
- Regulation:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Allison Kriel, Shaun R Brinsmade, Jessica L Tse, Ashley K Tehranchi, Alycia N Bittner, Abraham L Sonenshein, Jue D Wang
GTP dysregulation in Bacillus subtilis cells lacking (p)ppGpp results in phenotypic amino acid auxotrophy and failure to adapt to nutrient downshift and regulate biosynthesis genes.
J Bacteriol: 2014, 196(1);189-201
[PubMed:24163341]
[WorldCat.org]
[DOI]
(I p)
Ulrike Mäder, Georg Homuth, Christian Scharf, Knut Büttner, Rüdiger Bode, Michael Hecker
Transcriptome and proteome analysis of Bacillus subtilis gene expression modulated by amino acid availability.
J Bacteriol: 2002, 184(15);4288-95
[PubMed:12107147]
[WorldCat.org]
[DOI]
(P p)
C Parsot
Evolution of biosynthetic pathways: a common ancestor for threonine synthase, threonine dehydratase and D-serine dehydratase.
EMBO J: 1986, 5(11);3013-9
[PubMed:3098560]
[WorldCat.org]
[DOI]
(P p)