Difference between revisions of "ThiG"
(→References) |
(→Expression and regulation) |
||
| Line 92: | Line 92: | ||
* '''[[Sigma factor]]:''' | * '''[[Sigma factor]]:''' | ||
| − | * '''Regulation:''' | + | * '''Regulation:''' |
| + | ** repressed by thiamine ([[Thi-box]]) {{PubMed| 16356850}} | ||
* '''Regulatory mechanism:''' | * '''Regulatory mechanism:''' | ||
Revision as of 14:15, 24 November 2009
- Description: hydroxyethylthiazole phosphate biosynthesis
| Gene name | thiG |
| Synonyms | yjbT |
| Essential | no |
| Product | thiamin thiazole synthase |
| Function | biosynthesis of thiamine |
| MW, pI | 26 kDa, 4.718 |
| Gene length, protein length | 768 bp, 256 aa |
| Immediate neighbours | thiS, thiF |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 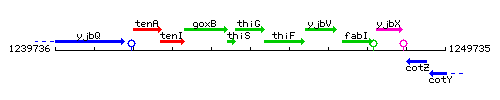
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU11690
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: thiG family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Cofactor(s):
- Effectors of protein activity:
- Localization: cytoplasm (according to Swiss-Prot)
Database entries
- Structure: 1XM3
- UniProt: O31618
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- repressed by thiamine (Thi-box) 16356850 PubMed
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Amrita Hazra, Abhishek Chatterjee, Tadhg P Begley
Biosynthesis of the thiamin thiazole in Bacillus subtilis: identification of the product of the thiazole synthase-catalyzed reaction.
J Am Chem Soc: 2009, 131(9);3225-9
[PubMed:19216519]
[WorldCat.org]
[DOI]
(I p)
Christine Eymann, Dörte Becher, Jörg Bernhardt, Katrin Gronau, Anja Klutzny, Michael Hecker
Dynamics of protein phosphorylation on Ser/Thr/Tyr in Bacillus subtilis.
Proteomics: 2007, 7(19);3509-26
[PubMed:17726680]
[WorldCat.org]
[DOI]
(P p)
Alain Lévine, Françoise Vannier, Cédric Absalon, Lauriane Kuhn, Peter Jackson, Elaine Scrivener, Valérie Labas, Joëlle Vinh, Patrick Courtney, Jérôme Garin, Simone J Séror
Analysis of the dynamic Bacillus subtilis Ser/Thr/Tyr phosphoproteome implicated in a wide variety of cellular processes.
Proteomics: 2006, 6(7);2157-73
[PubMed:16493705]
[WorldCat.org]
[DOI]
(P p)
Ethan C Settembre, Pieter C Dorrestein, Huili Zhai, Abhishek Chatterjee, Fred W McLafferty, Tadhg P Begley, Steven E Ealick
Thiamin biosynthesis in Bacillus subtilis: structure of the thiazole synthase/sulfur carrier protein complex.
Biochemistry: 2004, 43(37);11647-57
[PubMed:15362849]
[WorldCat.org]
[DOI]
(P p)
Joo-Heon Park, Pieter C Dorrestein, Huili Zhai, Cynthia Kinsland, Fred W McLafferty, Tadhg P Begley
Biosynthesis of the thiazole moiety of thiamin pyrophosphate (vitamin B1).
Biochemistry: 2003, 42(42);12430-8
[PubMed:14567704]
[WorldCat.org]
[DOI]
(P p)