Difference between revisions of "ThiC"
(→Categories containing this gene/protein) |
|||
| Line 85: | Line 85: | ||
* '''Domains:''' | * '''Domains:''' | ||
| − | * '''Modification:''' phosphorylated on Ser-565, Ser-586 and Tyr-589 {{PubMed|20509597}} | + | * '''Modification:''' |
| + | ** phosphorylated on Arg-556 {{PubMed|22517742}} | ||
| + | ** phosphorylated on Ser-565, Ser-586 and Tyr-589 {{PubMed|20509597}} | ||
* '''Cofactor(s):''' | * '''Cofactor(s):''' | ||
| Line 147: | Line 149: | ||
==Original publications== | ==Original publications== | ||
| − | <pubmed>16291685 9370266 20509597 12376536</pubmed> | + | <pubmed>16291685 9370266 20509597 12376536 22517742</pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 17:42, 21 April 2012
- Description: biosynthesis of the pyrimidine moiety of thiamine
| Gene name | thiC |
| Synonyms | thiA |
| Essential | no |
| Product | unknown |
| Function | biosynthesis of thiamine |
| Metabolic function and regulation of this protein in SubtiPathways: Thiamin | |
| MW, pI | 65 kDa, 5.262 |
| Gene length, protein length | 1770 bp, 590 aa |
| Immediate neighbours | ygaJ, ygaK |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 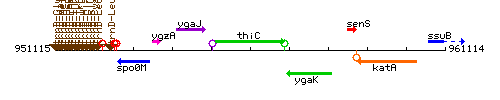
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed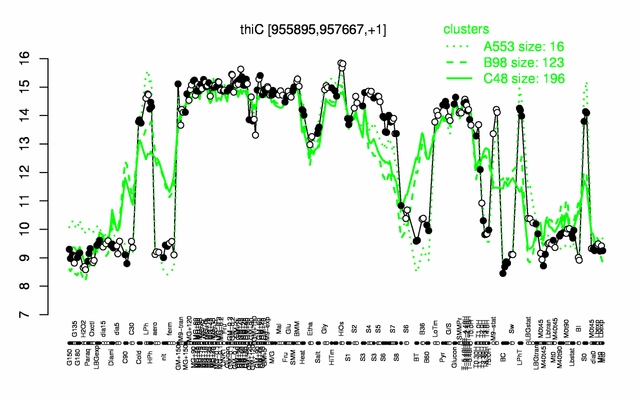
| |
Contents
Categories containing this gene/protein
biosynthesis of cofactors, phosphoproteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU08790
Phenotypes of a mutant
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: thiC family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- Structure: 1G6C
- UniProt: P45740
- KEGG entry: [2]
- E.C. number:
Additional information
- subject to Clp-dependent proteolysis upon glucose starvation PubMed
Expression and regulation
- Operon:
- Sigma factor:
- Regulation:
- Regulatory mechanism:
- Additional information: subject to Clp-dependent proteolysis upon glucose starvation PubMed
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system: B. pertussis adenylate cyclase-based bacterial two hybrid system (BACTH), available in Stülke lab
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Christopher T Jurgenson, Tadhg P Begley, Steven E Ealick
The structural and biochemical foundations of thiamin biosynthesis.
Annu Rev Biochem: 2009, 78;569-603
[PubMed:19348578]
[WorldCat.org]
[DOI]
(I p)
T P Begley, D M Downs, S E Ealick, F W McLafferty, A P Van Loon, S Taylor, N Campobasso, H J Chiu, C Kinsland, J J Reddick, J Xi
Thiamin biosynthesis in prokaryotes.
Arch Microbiol: 1999, 171(5);293-300
[PubMed:10382260]
[WorldCat.org]
[DOI]
(P p)
Original publications
Alexander K W Elsholz, Kürsad Turgay, Stephan Michalik, Bernd Hessling, Katrin Gronau, Dan Oertel, Ulrike Mäder, Jörg Bernhardt, Dörte Becher, Michael Hecker, Ulf Gerth
Global impact of protein arginine phosphorylation on the physiology of Bacillus subtilis.
Proc Natl Acad Sci U S A: 2012, 109(19);7451-6
[PubMed:22517742]
[WorldCat.org]
[DOI]
(I p)
Boumediene Soufi, Chanchal Kumar, Florian Gnad, Matthias Mann, Ivan Mijakovic, Boris Macek
Stable isotope labeling by amino acids in cell culture (SILAC) applied to quantitative proteomics of Bacillus subtilis.
J Proteome Res: 2010, 9(7);3638-46
[PubMed:20509597]
[WorldCat.org]
[DOI]
(I p)
Ghislain Schyns, Sébastien Potot, Yi Geng, Teresa M Barbosa, Adriano Henriques, John B Perkins
Isolation and characterization of new thiamine-deregulated mutants of Bacillus subtilis.
J Bacteriol: 2005, 187(23);8127-36
[PubMed:16291685]
[WorldCat.org]
[DOI]
(P p)
Dmitry A Rodionov, Alexey G Vitreschak, Andrey A Mironov, Mikhail S Gelfand
Comparative genomics of thiamin biosynthesis in procaryotes. New genes and regulatory mechanisms.
J Biol Chem: 2002, 277(50);48949-59
[PubMed:12376536]
[WorldCat.org]
[DOI]
(P p)
Y Zhang, T P Begley
Cloning, sequencing and regulation of thiA, a thiamin biosynthesis gene from Bacillus subtilis.
Gene: 1997, 198(1-2);73-82
[PubMed:9370266]
[WorldCat.org]
[DOI]
(P p)