Difference between revisions of "ThiC"
| Line 68: | Line 68: | ||
* '''Domains:''' | * '''Domains:''' | ||
| − | * '''Modification:''' | + | * '''Modification:''' phosphorylated on Ser-565 and Ser-586 {{PubMed|20509597}} |
* '''Cofactor(s):''' | * '''Cofactor(s):''' | ||
| Line 126: | Line 126: | ||
<pubmed>19348578 </pubmed> | <pubmed>19348578 </pubmed> | ||
==Original publications== | ==Original publications== | ||
| − | <pubmed>16291685 9370266 </pubmed> | + | <pubmed>16291685 9370266 20509597 </pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 08:11, 3 June 2010
- Description: biosynthesis of the pyrimidine moiety of thiamine
| Gene name | thiC |
| Synonyms | thiA |
| Essential | no |
| Product | unknown |
| Function | biosynthesis of thiamine |
| Metabolic function and regulation of this protein in SubtiPathways: Thiamin | |
| MW, pI | 65 kDa, 5.262 |
| Gene length, protein length | 1770 bp, 590 aa |
| Immediate neighbours | ygaJ, ygaK |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 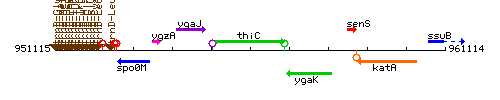
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU08790
Phenotypes of a mutant
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: thiC family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification: phosphorylated on Ser-565 and Ser-586 PubMed
- Cofactor(s):
- Effectors of protein activity:
- Interactions:
- Localization:
Database entries
- Structure: 1G6C
- UniProt: P45740
- KEGG entry: [2]
- E.C. number:
Additional information
- subject to Clp-dependent proteolysis upon glucose starvation PubMed
Expression and regulation
- Operon:
- Regulation:
- repressed by thiamine and 2-methyl-4-amino-5-hydroxymethylpyrimidine PubMed
- Regulatory mechanism:
- Additional information: subject to Clp-dependent proteolysis upon glucose starvation PubMed
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Christopher T Jurgenson, Tadhg P Begley, Steven E Ealick
The structural and biochemical foundations of thiamin biosynthesis.
Annu Rev Biochem: 2009, 78;569-603
[PubMed:19348578]
[WorldCat.org]
[DOI]
(I p)
Original publications
Boumediene Soufi, Chanchal Kumar, Florian Gnad, Matthias Mann, Ivan Mijakovic, Boris Macek
Stable isotope labeling by amino acids in cell culture (SILAC) applied to quantitative proteomics of Bacillus subtilis.
J Proteome Res: 2010, 9(7);3638-46
[PubMed:20509597]
[WorldCat.org]
[DOI]
(I p)
Ghislain Schyns, Sébastien Potot, Yi Geng, Teresa M Barbosa, Adriano Henriques, John B Perkins
Isolation and characterization of new thiamine-deregulated mutants of Bacillus subtilis.
J Bacteriol: 2005, 187(23);8127-36
[PubMed:16291685]
[WorldCat.org]
[DOI]
(P p)
Y Zhang, T P Begley
Cloning, sequencing and regulation of thiA, a thiamin biosynthesis gene from Bacillus subtilis.
Gene: 1997, 198(1-2);73-82
[PubMed:9370266]
[WorldCat.org]
[DOI]
(P p)