Difference between revisions of "TagE"
| Line 87: | Line 87: | ||
=Expression and regulation= | =Expression and regulation= | ||
| + | * '''Operon:''' ''[[tagD]]-[[tagE]]-[[tagF]]'' {{PubMed|2507871}} | ||
| − | + | * '''[[Sigma factor]]:''' [[SigA]] {{PubMed|7581998}} | |
| − | |||
| − | * '''[[Sigma factor]]:''' | ||
* '''Regulation:''' | * '''Regulation:''' | ||
| − | ** repressed | + | ** repressed by phosphate ([[PhoP]]) [http://www.ncbi.nlm.nih.gov/sites/entrez/9457886 PubMed] |
* '''Regulatory mechanism:''' | * '''Regulatory mechanism:''' | ||
** [[PhoP]]: transcription repression [http://www.ncbi.nlm.nih.gov/sites/entrez/9457886 PubMed] | ** [[PhoP]]: transcription repression [http://www.ncbi.nlm.nih.gov/sites/entrez/9457886 PubMed] | ||
| + | ** [[WalR]]: transcription activation {{PubMed|12950927}} | ||
* '''Additional information:''' | * '''Additional information:''' | ||
| Line 120: | Line 120: | ||
=References= | =References= | ||
| − | <pubmed>9457886,, | + | <pubmed>9457886,7581998,2507871,17218307,12950927 </pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 21:17, 4 January 2010
- Description: UDP-glucose:polyglycerol phosphate glucosyltransferase
| Gene name | tagE |
| Synonyms | rodD, gtaA, gtaD |
| Essential | no |
| Product | UDP-glucose:polyglycerol phosphate glucosyltransferase |
| Function | biosynthesis of teichoic acid |
| MW, pI | 78 kDa, 6.026 |
| Gene length, protein length | 2019 bp, 673 aa |
| Immediate neighbours | tagF, tagD |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 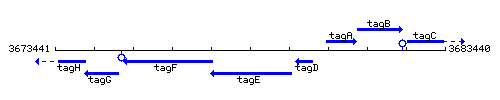
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU35730
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: UDP-glucose + poly(glycerol phosphate) = UDP + O-(alpha-D-glucosyl)poly(glycerol phosphate) (according to Swiss-Prot)
- Protein family: glycosyltransferase 1 family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification: phosphorylation on Ser-2 PubMed
- Cofactor(s):
- Effectors of protein activity:
- Interactions:
- Localization: cytoplasm (according to Swiss-Prot)
Database entries
- Structure:
- UniProt: P13484
- KEGG entry: [3]
- E.C. number: 2.4.1.52
Additional information
Expression and regulation
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Boris Macek, Ivan Mijakovic, Jesper V Olsen, Florian Gnad, Chanchal Kumar, Peter R Jensen, Matthias Mann
The serine/threonine/tyrosine phosphoproteome of the model bacterium Bacillus subtilis.
Mol Cell Proteomics: 2007, 6(4);697-707
[PubMed:17218307]
[WorldCat.org]
[DOI]
(P p)
Alistair Howell, Sarah Dubrac, Kasper Krogh Andersen, David Noone, Juliette Fert, Tarek Msadek, Kevin Devine
Genes controlled by the essential YycG/YycF two-component system of Bacillus subtilis revealed through a novel hybrid regulator approach.
Mol Microbiol: 2003, 49(6);1639-55
[PubMed:12950927]
[WorldCat.org]
[DOI]
(P p)
W Liu, S Eder, F M Hulett
Analysis of Bacillus subtilis tagAB and tagDEF expression during phosphate starvation identifies a repressor role for PhoP-P.
J Bacteriol: 1998, 180(3);753-8
[PubMed:9457886]
[WorldCat.org]
[DOI]
(P p)
C Mauël, A Bauduret, C Chervet, S Beggah, D Karamata
In Bacillus subtilis 168, teichoic acid of the cross-wall may be different from that of the cylinder: a hypothesis based on transcription analysis of tag genes.
Microbiology (Reading): 1995, 141 ( Pt 10);2379-89
[PubMed:7581998]
[WorldCat.org]
[DOI]
(P p)
A L Honeyman, G C Stewart
The nucleotide sequence of the rodC operon of Bacillus subtilis.
Mol Microbiol: 1989, 3(9);1257-68
[PubMed:2507871]
[WorldCat.org]
[DOI]
(P p)