SpoIIIAH
- Description: component of the SpoIIIA-SpoIIQ type III secretion system residing in the forespore membrane, required for SigG activation
| Gene name | spoIIIAH |
| Synonyms | |
| Essential | no |
| Product | part of the transmembrane channel linking the mother cell and the forespore |
| Function | activation of SigG, forespore encasement by the spore coat |
| Gene expression levels in SubtiExpress: spoIIIAH | |
| Interactions involving this protein in SubtInteract: SpoIIIAH | |
| MW, pI | 23 kDa, 4.627 |
| Gene length, protein length | 654 bp, 218 aa |
| Immediate neighbours | accB, spoIIIAG |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed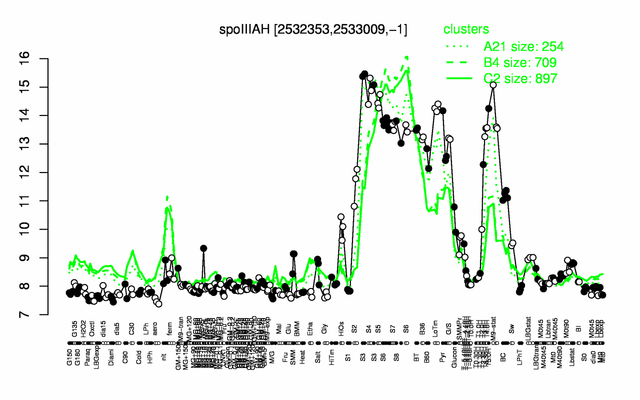
| |
Contents
Categories containing this gene/protein
protein secretion, sporulation proteins, membrane proteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU24360
Phenotypes of a mutant
- block of sporulation after engulfment
Database entries
- BsubCyc: BSU24360
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- required for forespore encasement by the spore coat PubMed
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- BsubCyc: BSU24360
- UniProt: P49785
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Operon:
- Regulation:
- Additional information: the internal promoter is essential for sporulation, it is twice as active as the promoter in front of spoIIIAA, suggesting that SpoIIIAG and SpoIIIAH are required in larger amounts as compared to the other products of the operon PubMed
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
- Charles Moran, Emory University, NC, USA homepage
Your additional remarks
References
Reviews
Adam D Crawshaw, Mónica Serrano, Will A Stanley, Adriano O Henriques, Paula S Salgado
A mother cell-to-forespore channel: current understanding and future challenges.
FEMS Microbiol Lett: 2014, 358(2);129-36
[PubMed:25105965]
[WorldCat.org]
[DOI]
(I p)
Lotte Søgaard-Andersen
Stably bridging a great divide: localization of the SpoIIQ landmark protein in Bacillus subtilis.
Mol Microbiol: 2013, 89(6);1019-24
[PubMed:23944268]
[WorldCat.org]
[DOI]
(I p)
Original publications
Jennifer Fredlund, Dan Broder, Tinya Fleming, Clémence Claussin, Kit Pogliano
The SpoIIQ landmark protein has different requirements for septal localization and immobilization.
Mol Microbiol: 2013, 89(6);1053-68
[PubMed:23859254]
[WorldCat.org]
[DOI]
(I p)
Christopher D A Rodrigues, Kathleen A Marquis, Jeffrey Meisner, David Z Rudner
Peptidoglycan hydrolysis is required for assembly and activity of the transenvelope secretion complex during sporulation in Bacillus subtilis.
Mol Microbiol: 2013, 89(6);1039-52
[PubMed:23834622]
[WorldCat.org]
[DOI]
(I p)
Jeffrey Meisner, Tatsuya Maehigashi, Ingemar André, Christine M Dunham, Charles P Moran
Structure of the basal components of a bacterial transporter.
Proc Natl Acad Sci U S A: 2012, 109(14);5446-51
[PubMed:22431613]
[WorldCat.org]
[DOI]
(I p)
Vladimir M Levdikov, Elena V Blagova, Amanda McFeat, Mark J Fogg, Keith S Wilson, Anthony J Wilkinson
Structure of components of an intercellular channel complex in sporulating Bacillus subtilis.
Proc Natl Acad Sci U S A: 2012, 109(14);5441-5
[PubMed:22431604]
[WorldCat.org]
[DOI]
(I p)
Peter T McKenney, Patrick Eichenberger
Dynamics of spore coat morphogenesis in Bacillus subtilis.
Mol Microbiol: 2012, 83(2);245-60
[PubMed:22171814]
[WorldCat.org]
[DOI]
(I p)
Jeffrey Meisner, Charles P Moran
A LytM domain dictates the localization of proteins to the mother cell-forespore interface during bacterial endospore formation.
J Bacteriol: 2011, 193(3);591-8
[PubMed:21097616]
[WorldCat.org]
[DOI]
(I p)
Thierry Doan, Cecile Morlot, Jeffrey Meisner, Monica Serrano, Adriano O Henriques, Charles P Moran, David Z Rudner
Novel secretion apparatus maintains spore integrity and developmental gene expression in Bacillus subtilis.
PLoS Genet: 2009, 5(7);e1000566
[PubMed:19609349]
[WorldCat.org]
[DOI]
(I p)
Jeffrey Meisner, Xin Wang, Monica Serrano, Adriano O Henriques, Charles P Moran
A channel connecting the mother cell and forespore during bacterial endospore formation.
Proc Natl Acad Sci U S A: 2008, 105(39);15100-5
[PubMed:18812514]
[WorldCat.org]
[DOI]
(I p)
Amy H Camp, Richard Losick
A novel pathway of intercellular signalling in Bacillus subtilis involves a protein with similarity to a component of type III secretion channels.
Mol Microbiol: 2008, 69(2);402-17
[PubMed:18485064]
[WorldCat.org]
[DOI]
(I p)
Chris Guillot, Charles P Moran
Essential internal promoter in the spoIIIA locus of Bacillus subtilis.
J Bacteriol: 2007, 189(20);7181-9
[PubMed:17693505]
[WorldCat.org]
[DOI]
(P p)
Shinobu Chiba, Kristina Coleman, Kit Pogliano
Impact of membrane fusion and proteolysis on SpoIIQ dynamics and interaction with SpoIIIAH.
J Biol Chem: 2007, 282(4);2576-86
[PubMed:17121846]
[WorldCat.org]
[DOI]
(P p)
Thierry Doan, Kathleen A Marquis, David Z Rudner
Subcellular localization of a sporulation membrane protein is achieved through a network of interactions along and across the septum.
Mol Microbiol: 2005, 55(6);1767-81
[PubMed:15752199]
[WorldCat.org]
[DOI]
(P p)
Bill Blaylock, Xin Jiang, Aileen Rubio, Charles P Moran, Kit Pogliano
Zipper-like interaction between proteins in adjacent daughter cells mediates protein localization.
Genes Dev: 2004, 18(23);2916-28
[PubMed:15574594]
[WorldCat.org]
[DOI]
(P p)
N Illing, J Errington
The spoIIIA operon of Bacillus subtilis defines a new temporal class of mother-cell-specific sporulation genes under the control of the sigma E form of RNA polymerase.
Mol Microbiol: 1991, 5(8);1927-40
[PubMed:1766372]
[WorldCat.org]
[DOI]
(P p)