SpoIIE
- Description: protein serine phosphatase, septum-associated PP2C, dephosphorylation of SpoIIAA
| Gene name | spoIIE |
| Synonyms | spoIIH, spoIIK |
| Essential | no |
| Product | protein serine phosphatase, septum-associated PP2C |
| Function | control of SigF activity required for normal formation of the asymmetric septum |
| Metabolic function and regulation of this protein in SubtiPathways: Stress | |
| MW, pI | 91 kDa, 6.137 |
| Gene length, protein length | 2481 bp, 827 aa |
| Immediate neighbours | trnSL-Glu1, yabS |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 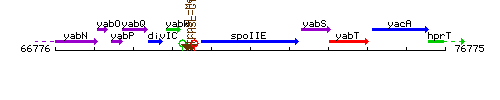
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU00640
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: A phosphoprotein + H2O = a protein + phosphate (according to Swiss-Prot) dephosphorylation of SpoIIAA
- Protein family: PP2C phosphatase
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization: cell membrane (according to Swiss-Prot)
Database entries
- Structure:
- Swiss prot entry: P37475
- KEGG entry: [3]
- E.C. number: 3.1.3.16
Additional information
Expression and regulation
- Operon:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Imrich Barak, Slovak Academy of Science, Bratislava, Slovakia homepage
Your additional remarks
References
Nathalie Campo, Kathleen A Marquis, David Z Rudner
SpoIIQ anchors membrane proteins on both sides of the sporulation septum in Bacillus subtilis.
J Biol Chem: 2008, 283(8);4975-82
[PubMed:18077456]
[WorldCat.org]
[DOI]
(P p)
Oleg A Igoshin, Chester W Price, Michael A Savageau
Signalling network with a bistable hysteretic switch controls developmental activation of the sigma transcription factor in Bacillus subtilis.
Mol Microbiol: 2006, 61(1);165-84
[PubMed:16824103]
[WorldCat.org]
[DOI]
(P p)
Shonna M McBride, Aileen Rubio, Lei Wang, William G Haldenwang
Contributions of protein structure and gene position to the compartmentalization of the regulatory proteins sigma(E) and SpoIIE in sporulating Bacillus subtilis.
Mol Microbiol: 2005, 57(2);434-51
[PubMed:15978076]
[WorldCat.org]
[DOI]
(P p)
Karen Carniol, Sigal Ben-Yehuda, Nicole King, Richard Losick
Genetic dissection of the sporulation protein SpoIIE and its role in asymmetric division in Bacillus subtilis.
J Bacteriol: 2005, 187(10);3511-20
[PubMed:15866939]
[WorldCat.org]
[DOI]
(P p)
Masaya Fujita, José Eduardo González-Pastor, Richard Losick
High- and low-threshold genes in the Spo0A regulon of Bacillus subtilis.
J Bacteriol: 2005, 187(4);1357-68
[PubMed:15687200]
[WorldCat.org]
[DOI]
(P p)
Karen Carniol, Tae-Jong Kim, Chester W Price, Richard Losick
Insulation of the sigmaF regulatory system in Bacillus subtilis.
J Bacteriol: 2004, 186(13);4390-4
[PubMed:15205443]
[WorldCat.org]
[DOI]
(P p)
Karen Carniol, Patrick Eichenberger, Richard Losick
A threshold mechanism governing activation of the developmental regulatory protein sigma F in Bacillus subtilis.
J Biol Chem: 2004, 279(15);14860-70
[PubMed:14744853]
[WorldCat.org]
[DOI]
(P p)
Andrea Feucht, Laura Abbotts, Jeffery Errington
The cell differentiation protein SpoIIE contains a regulatory site that controls its phosphatase activity in response to asymmetric septation.
Mol Microbiol: 2002, 45(4);1119-30
[PubMed:12180929]
[WorldCat.org]
[DOI]
(P p)
Sigal Ben-Yehuda, Richard Losick
Asymmetric cell division in B. subtilis involves a spiral-like intermediate of the cytokinetic protein FtsZ.
Cell: 2002, 109(2);257-66
[PubMed:12007411]
[WorldCat.org]
[DOI]
(P p)
J Clarkson, I D Campbell, M D Yudkin
NMR studies of the interactions of SpoIIAA with its partner proteins that regulate sporulation in Bacillus subtilis.
J Mol Biol: 2001, 314(3);359-64
[PubMed:11846550]
[WorldCat.org]
[DOI]
(P p)
I Lucet, A Feucht, M D Yudkin, J Errington
Direct interaction between the cell division protein FtsZ and the cell differentiation protein SpoIIE.
EMBO J: 2000, 19(7);1467-75
[PubMed:10747015]
[WorldCat.org]
[DOI]
(P p)
A Feucht, R A Daniel, J Errington
Characterization of a morphological checkpoint coupling cell-specific transcription to septation in Bacillus subtilis.
Mol Microbiol: 1999, 33(5);1015-26
[PubMed:10476035]
[WorldCat.org]
[DOI]
(P p)
N King, O Dreesen, P Stragier, K Pogliano, R Losick
Septation, dephosphorylation, and the activation of sigmaF during sporulation in Bacillus subtilis.
Genes Dev: 1999, 13(9);1156-67
[PubMed:10323866]
[WorldCat.org]
[DOI]
(P p)
I Lucet, R Borriss, M D Yudkin
Purification, kinetic properties, and intracellular concentration of SpoIIE, an integral membrane protein that regulates sporulation in Bacillus subtilis.
J Bacteriol: 1999, 181(10);3242-5
[PubMed:10322028]
[WorldCat.org]
[DOI]
(P p)
P J Lewis, L J Wu, J Errington
Establishment of prespore-specific gene expression in Bacillus subtilis: localization of SpoIIE phosphatase and initiation of compartment-specific proteolysis.
J Bacteriol: 1998, 180(13);3276-84
[PubMed:9642177]
[WorldCat.org]
[DOI]
(P p)
L J Wu, A Feucht, J Errington
Prespore-specific gene expression in Bacillus subtilis is driven by sequestration of SpoIIE phosphatase to the prespore side of the asymmetric septum.
Genes Dev: 1998, 12(9);1371-80
[PubMed:9573053]
[WorldCat.org]
[DOI]
(P p)
A Khvorova, L Zhang, M L Higgins, P J Piggot
The spoIIE locus is involved in the Spo0A-dependent switch in the location of FtsZ rings in Bacillus subtilis.
J Bacteriol: 1998, 180(5);1256-60
[PubMed:9495766]
[WorldCat.org]
[DOI]
(P p)
P A Levin, R Losick, P Stragier, F Arigoni
Localization of the sporulation protein SpoIIE in Bacillus subtilis is dependent upon the cell division protein FtsZ.
Mol Microbiol: 1997, 25(5);839-46
[PubMed:9364910]
[WorldCat.org]
[DOI]
(P p)
K Pogliano, A E Hofmeister, R Losick
Disappearance of the sigma E transcription factor from the forespore and the SpoIIE phosphatase from the mother cell contributes to establishment of cell-specific gene expression during sporulation in Bacillus subtilis.
J Bacteriol: 1997, 179(10);3331-41
[PubMed:9150232]
[WorldCat.org]
[DOI]
(P p)
P J Lewis, T Magnin, J Errington
Compartmentalized distribution of the proteins controlling the prespore-specific transcription factor sigmaF of Bacillus subtilis.
Genes Cells: 1996, 1(10);881-94
[PubMed:9077448]
[WorldCat.org]
[DOI]
(P p)
F Arigoni, L Duncan, S Alper, R Losick, P Stragier
SpoIIE governs the phosphorylation state of a protein regulating transcription factor sigma F during sporulation in Bacillus subtilis.
Proc Natl Acad Sci U S A: 1996, 93(8);3238-42
[PubMed:8622920]
[WorldCat.org]
[DOI]
(P p)
A Feucht, T Magnin, M D Yudkin, J Errington
Bifunctional protein required for asymmetric cell division and cell-specific transcription in Bacillus subtilis.
Genes Dev: 1996, 10(7);794-803
[PubMed:8846916]
[WorldCat.org]
[DOI]
(P p)
L Duncan, S Alper, F Arigoni, R Losick, P Stragier
Activation of cell-specific transcription by a serine phosphatase at the site of asymmetric division.
Science: 1995, 270(5236);641-4
[PubMed:7570023]
[WorldCat.org]
[DOI]
(P p)
F Arigoni, K Pogliano, C D Webb, P Stragier, R Losick
Localization of protein implicated in establishment of cell type to sites of asymmetric division.
Science: 1995, 270(5236);637-40
[PubMed:7570022]
[WorldCat.org]
[DOI]
(P p)
K York, T J Kenney, S Satola, C P Moran, H Poth, P Youngman
Spo0A controls the sigma A-dependent activation of Bacillus subtilis sporulation-specific transcription unit spoIIE.
J Bacteriol: 1992, 174(8);2648-58
[PubMed:1556084]
[WorldCat.org]
[DOI]
(P p)
P Margolis, A Driks, R Losick
Establishment of cell type by compartmentalized activation of a transcription factor.
Science: 1991, 254(5031);562-5
[PubMed:1948031]
[WorldCat.org]
[DOI]
(P p)