Difference between revisions of "SodA"
(→Extended information on the protein) |
|||
| Line 83: | Line 83: | ||
* '''[[SubtInteract|Interactions]]:''' | * '''[[SubtInteract|Interactions]]:''' | ||
| − | * '''[[Localization]]:''' | + | * '''[[Localization]]:''' cytoplasm {{PubMed|22174384}} |
=== Database entries === | === Database entries === | ||
| Line 103: | Line 103: | ||
* '''[[Sigma factor]]:''' [[SigB]] [http://www.ncbi.nlm.nih.gov/pubmed/15805528 PubMed] | * '''[[Sigma factor]]:''' [[SigB]] [http://www.ncbi.nlm.nih.gov/pubmed/15805528 PubMed] | ||
| − | * '''Regulation:''' induced by stress ([[SigB]]) [http://www.ncbi.nlm.nih.gov/pubmed/15805528 PubMed] | + | * '''Regulation:''' |
| + | ** induced by stress ([[SigB]]) [http://www.ncbi.nlm.nih.gov/pubmed/15805528 PubMed] | ||
| + | ** constitutive expression {{PubMed|22174384}} | ||
* '''Regulatory mechanism:''' | * '''Regulatory mechanism:''' | ||
| Line 130: | Line 132: | ||
=References= | =References= | ||
| − | <pubmed>9393707,9573176, 20525796,15805528 17218307, 9658017 10074093 </pubmed> | + | <pubmed>9393707,9573176, 20525796,15805528 17218307, 9658017 10074093 22174384</pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 10:10, 20 December 2011
- Description: superoxide dismutase, general stress protein, important for survival of ethanol stress and at low temperatures
| Gene name | sodA |
| Synonyms | yqgD |
| Essential | no |
| Product | superoxide dismutase |
| Function | detoxification of oxygen radicals |
| MW, pI | 22 kDa, 5.203 |
| Gene length, protein length | 606 bp, 202 aa |
| Immediate neighbours | yqgE, yqgC |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 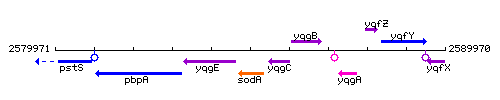
This image was kindly provided by SubtiList
| |
Contents
Categories containing this gene/protein
general stress proteins (controlled by SigB), resistance against oxidative and electrophile stress, membrane proteins, phosphoproteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU25020
Phenotypes of a mutant
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: 2 superoxide + 2 H+ = O2 + H2O2 (according to Swiss-Prot)
- Protein family: iron/manganese superoxide dismutase family (according to Swiss-Prot)
- Paralogous protein(s): SodF
Extended information on the protein
- Kinetic information:
- Domains:
- Modification: phosphorylation on Thr-34 AND Thr-70 PubMed
- Cofactor(s): manganese
- Effectors of protein activity:
- Localization: cytoplasm PubMed
Database entries
- Structure: 2RCV
- UniProt: P54375
- KEGG entry: [2]
- E.C. number:
Additional information
Expression and regulation
- Operon:
- Regulatory mechanism:
- Additional information:
- The mRNA has a long 5' leader region. This may indicate RNA-based regulation PubMed
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Wang Yung Tu, Susanne Pohl, Pijug Summpunn, Silvio Hering, Sandra Kerstan, Colin R Harwood
Comparative analysis of the responses of related pathogenic and environmental bacteria to oxidative stress.
Microbiology (Reading): 2012, 158(Pt 3);636-647
[PubMed:22174384]
[WorldCat.org]
[DOI]
(I p)
Irnov Irnov, Cynthia M Sharma, Jörg Vogel, Wade C Winkler
Identification of regulatory RNAs in Bacillus subtilis.
Nucleic Acids Res: 2010, 38(19);6637-51
[PubMed:20525796]
[WorldCat.org]
[DOI]
(I p)
Boris Macek, Ivan Mijakovic, Jesper V Olsen, Florian Gnad, Chanchal Kumar, Peter R Jensen, Matthias Mann
The serine/threonine/tyrosine phosphoproteome of the model bacterium Bacillus subtilis.
Mol Cell Proteomics: 2007, 6(4);697-707
[PubMed:17218307]
[WorldCat.org]
[DOI]
(P p)
Dirk Höper, Uwe Völker, Michael Hecker
Comprehensive characterization of the contribution of individual SigB-dependent general stress genes to stress resistance of Bacillus subtilis.
J Bacteriol: 2005, 187(8);2810-26
[PubMed:15805528]
[WorldCat.org]
[DOI]
(P p)
T Inaoka, Y Matsumura, T Tsuchido
SodA and manganese are essential for resistance to oxidative stress in growing and sporulating cells of Bacillus subtilis.
J Bacteriol: 1999, 181(6);1939-43
[PubMed:10074093]
[WorldCat.org]
[DOI]
(P p)
T Inaoka, Y Matsumura, T Tsuchido
Molecular cloning and nucleotide sequence of the superoxide dismutase gene and characterization of its product from Bacillus subtilis.
J Bacteriol: 1998, 180(14);3697-703
[PubMed:9658017]
[WorldCat.org]
[DOI]
(P p)
A O Henriques, L R Melsen, C P Moran
Involvement of superoxide dismutase in spore coat assembly in Bacillus subtilis.
J Bacteriol: 1998, 180(9);2285-91
[PubMed:9573176]
[WorldCat.org]
[DOI]
(P p)
L Casillas-Martinez, P Setlow
Alkyl hydroperoxide reductase, catalase, MrgA, and superoxide dismutase are not involved in resistance of Bacillus subtilis spores to heat or oxidizing agents.
J Bacteriol: 1997, 179(23);7420-5
[PubMed:9393707]
[WorldCat.org]
[DOI]
(P p)