Difference between revisions of "SinR"
(→Biological materials) |
(→Biological materials) |
||
| Line 118: | Line 118: | ||
=Biological materials = | =Biological materials = | ||
| − | * '''Mutant:''' TMB079 ''sinR::spec'', available in [[Stülke]] lab | + | * '''Mutant:''' TMB079 ''sinR::spec'', GP736 (tetR), available in [[Stülke]] lab |
* '''Expression vector:''' | * '''Expression vector:''' | ||
Revision as of 09:56, 11 February 2010
- Description: transcriptional regulator of post-exponential-phase responses genes
| Gene name | sinR |
| Synonyms | sin, flaD |
| Essential | no |
| Product | transcriptional regulator of post-exponential-phase responses genes |
| Function | regulation of post-exponential gene expression |
| Metabolic function and regulation of this protein in SubtiPathways: Central C-metabolism | |
| MW, pI | 12 kDa, 7.177 |
| Gene length, protein length | 333 bp, 111 aa |
| Immediate neighbours | sinI, tasA |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 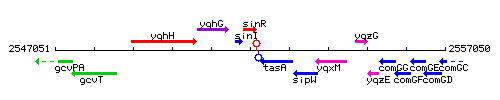
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU24610
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family:
- Paralogous protein(s): SlrR
Genes controlled by SinR
- Activation by SinR: comK
- Repression by SinR: aprE, epsA-epsB-epsC-epsD-epsE-epsF-epsG-epsH-epsI-epsJ-epsK-epsL-epsM-epsN-epsO PubMed,kinB, rapG PubMed, sigD, spo0A,spoIIAA-spoIIAB-sigF, spoIIE, spoIIGA-sigE-sigG, spoVG PubMed, yqxM-sipW-tasA PubMed, yvgN PubMed, ywbD PubMed
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Localization:
Database entries
- Structure: 1B0N (complex SinR-SinI)
- UniProt: P06533
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant: TMB079 sinR::spec, GP736 (tetR), available in Stülke lab
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Prashant Kodgire, K Krishnamurthy Rao
A dual mode of regulation of flgM by ScoC in Bacillus subtilis.
Can J Microbiol: 2009, 55(8);983-9
[PubMed:19898538]
[WorldCat.org]
[DOI]
(I p)
Yunrong Chai, Roberto Kolter, Richard Losick
Paralogous antirepressors acting on the master regulator for biofilm formation in Bacillus subtilis.
Mol Microbiol: 2009, 74(4);876-87
[PubMed:19788541]
[WorldCat.org]
[DOI]
(I p)
Yunrong Chai, Roberto Kolter, Richard Losick
A widely conserved gene cluster required for lactate utilization in Bacillus subtilis and its involvement in biofilm formation.
J Bacteriol: 2009, 191(8);2423-30
[PubMed:19201793]
[WorldCat.org]
[DOI]
(I p)
Frances Chu, Daniel B Kearns, Anna McLoon, Yunrong Chai, Roberto Kolter, Richard Losick
A novel regulatory protein governing biofilm formation in Bacillus subtilis.
Mol Microbiol: 2008, 68(5);1117-27
[PubMed:18430133]
[WorldCat.org]
[DOI]
(I p)
Yunrong Chai, Frances Chu, Roberto Kolter, Richard Losick
Bistability and biofilm formation in Bacillus subtilis.
Mol Microbiol: 2008, 67(2);254-63
[PubMed:18047568]
[WorldCat.org]
[DOI]
(P p)
Prashant Kodgire, Madhulika Dixit, K Krishnamurthy Rao
ScoC and SinR negatively regulate epr by corepression in Bacillus subtilis.
J Bacteriol: 2006, 188(17);6425-8
[PubMed:16923912]
[WorldCat.org]
[DOI]
(P p)
Steven S Branda, Frances Chu, Daniel B Kearns, Richard Losick, Roberto Kolter
A major protein component of the Bacillus subtilis biofilm matrix.
Mol Microbiol: 2006, 59(4);1229-38
[PubMed:16430696]
[WorldCat.org]
[DOI]
(P p)
Frances Chu, Daniel B Kearns, Steven S Branda, Roberto Kolter, Richard Losick
Targets of the master regulator of biofilm formation in Bacillus subtilis.
Mol Microbiol: 2006, 59(4);1216-28
[PubMed:16430695]
[WorldCat.org]
[DOI]
(P p)
Daniel B Kearns, Frances Chu, Steven S Branda, Roberto Kolter, Richard Losick
A master regulator for biofilm formation by Bacillus subtilis.
Mol Microbiol: 2005, 55(3);739-49
[PubMed:15661000]
[WorldCat.org]
[DOI]
(P p)
Alejandro Sánchez, Jorge Olmos
Bacillus subtilis transcriptional regulators interaction.
Biotechnol Lett: 2004, 26(5);403-7
[PubMed:15104138]
[WorldCat.org]
[DOI]
(P p)
Sasha H Shafikhani, Ines Mandic-Mulec, Mark A Strauch, Issar Smith, Terrance Leighton
Postexponential regulation of sin operon expression in Bacillus subtilis.
J Bacteriol: 2002, 184(2);564-71
[PubMed:11751836]
[WorldCat.org]
[DOI]
(P p)
D J Scott, S Leejeerajumnean, J A Brannigan, R J Lewis, A J Wilkinson, J G Hoggett
Quaternary re-arrangement analysed by spectral enhancement: the interaction of a sporulation repressor with its antagonist.
J Mol Biol: 1999, 293(5);997-1004
[PubMed:10547280]
[WorldCat.org]
[DOI]
(P p)
R J Lewis, J A Brannigan, W A Offen, I Smith, A J Wilkinson
An evolutionary link between sporulation and prophage induction in the structure of a repressor:anti-repressor complex.
J Mol Biol: 1998, 283(5);907-12
[PubMed:9799632]
[WorldCat.org]
[DOI]
(P p)
K Fredrick, J D Helmann
FlgM is a primary regulator of sigmaD activity, and its absence restores motility to a sinR mutant.
J Bacteriol: 1996, 178(23);7010-3
[PubMed:8955328]
[WorldCat.org]
[DOI]
(P p)
J Hahn, A Luttinger, D Dubnau
Regulatory inputs for the synthesis of ComK, the competence transcription factor of Bacillus subtilis.
Mol Microbiol: 1996, 21(4);763-75
[PubMed:8878039]
[WorldCat.org]
[DOI]
(P p)
M A Strauch
In vitro binding affinity of the Bacillus subtilis AbrB protein to six different DNA target regions.
J Bacteriol: 1995, 177(15);4532-6
[PubMed:7635837]
[WorldCat.org]
[DOI]
(P p)
P T Kallio, J E Fagelson, J A Hoch, M A Strauch
The transition state regulator Hpr of Bacillus subtilis is a DNA-binding protein.
J Biol Chem: 1991, 266(20);13411-7
[PubMed:1906467]
[WorldCat.org]
(P p)
N K Gaur, K Cabane, I Smith
Structure and expression of the Bacillus subtilis sin operon.
J Bacteriol: 1988, 170(3);1046-53
[PubMed:3125149]
[WorldCat.org]
[DOI]
(P p)