Difference between revisions of "SecA"
(→Extended information on the protein) |
(→Basic information/ Evolution) |
||
| Line 54: | Line 54: | ||
=== Basic information/ Evolution === | === Basic information/ Evolution === | ||
| − | * '''Catalyzed reaction/ biological activity:''' | + | * '''Catalyzed reaction/ biological activity:''' ATP -> ADP + Pi + preprotein translocation |
| − | * '''Protein family:''' | + | * '''Protein family:''' SecA family (according to Swiss-Prot) |
| − | * '''Paralogous protein(s):''' | + | * '''Paralogous protein(s):''' none in Bacillus, some species have a paralogous secA gene named secA2 that has an altered substrate range |
=== Extended information on the protein === | === Extended information on the protein === | ||
Revision as of 11:42, 2 June 2009
- Description: preprotein translocase subunit (ATPase)
| Gene name | secA |
| Synonyms | div, div-341, ts-341 |
| Essential | yes PubMed |
| Product | preprotein translocase subunit (ATPase) |
| Function | protein secretion |
| MW, pI | 95 kDa, 5.34 |
| Gene length, protein length | 2523 bp, 841 aa |
| Immediate neighbours | prfB, yvyD |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 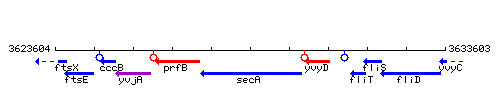
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Coordinates:
Phenotypes of a mutant
essential PubMed
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: ATP -> ADP + Pi + preprotein translocation
- Protein family: SecA family (according to Swiss-Prot)
- Paralogous protein(s): none in Bacillus, some species have a paralogous secA gene named secA2 that has an altered substrate range
Extended information on the protein
- Kinetic information:
- Domains: nucleotide binding domain, preprotein binding domain, IRA2 domain, scaffold domain, wing domain, IRA1 domain, C-terminal domain
- Modification:
- Cofactor(s): magnesium
- Effectors of protein activity: anionic phospholipids, preprotein, SecY
- Localization: cell membrane (according to Swiss-Prot)
Database entries
- Swiss prot entry: P28366
- KEGG entry: BSU35300
- E.C. number:
Additional information
- subject to Clp-dependent proteolysis upon glucose starvation PubMed
Expression and regulation
- Regulation:
- Regulatory mechanism:
- Additional information: subject to Clp-dependent proteolysis upon glucose starvation PubMed
Biological materials
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody: