SdhC
- Description: succinate dehydrogenase (cytochrome b558 subunit)
| Gene name | sdhC |
| Synonyms | |
| Essential | no |
| Product | succinate dehydrogenase (cytochrome b558 subunit) |
| Function | TCA cycle |
| Gene expression levels in SubtiExpress: sdhC | |
| Interactions involving this protein in SubtInteract: SdhC | |
| Metabolic function and regulation of this protein in SubtiPathways: Central C-metabolism | |
| MW, pI | 22 kDa, 9.831 |
| Gene length, protein length | 606 bp, 202 aa |
| Immediate neighbours | sdhA, yslB |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 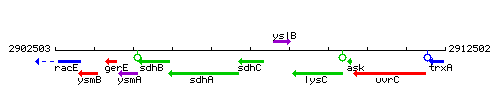
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed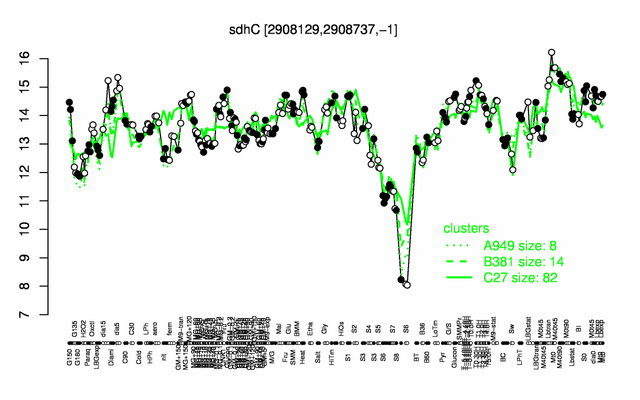
| |
Contents
Categories containing this gene/protein
carbon core metabolism, membrane proteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU28450
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: cytochrome b558 family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s): Fe
- Effectors of protein activity:
- Localization:
- membrane protein PubMed
Database entries
- Structure: 1NEK (E. coli)
- UniProt: P08064
- KEGG entry: [3]
- E.C. number: 1.3.99.1
Additional information
- This enzyme is a trimer membrane-bound PubMed PubMed
- One subunit is bound to citochrome b558, and this subunit is the one bound to the cytosolic side of the membrane PubMed PubMed
- Another subunit is the flavoprotein one, required for FAD usage PubMed PubMed
- The other subunit has an iron-sulphur domain necessary for the catalytic activity PubMed PubMed
- extensive information on the structure and enzymatic properties of succinate dehydrogenase can be found at Proteopedia
Expression and regulation
- Regulation:
- Additional information:
Biological materials
- Mutant: GP743 (sdhCA, cat), available in Stülke lab
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system: B. pertussis adenylate cyclase-based bacterial two hybrid system (BACTH), available in Stülke lab
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Lars Hederstedt
Succinate:quinone oxidoreductase in the bacteria Paracoccus denitrificans and Bacillus subtilis.
Biochim Biophys Acta: 2002, 1553(1-2);74-83
[PubMed:11803018]
[WorldCat.org]
[DOI]
(P p)
Original publications
Pedro M F Sousa, Marco A M Videira, Filipe A S Santos, Brian L Hood, Thomas P Conrads, Ana M P Melo
The bc:caa3 supercomplexes from the Gram positive bacterium Bacillus subtilis respiratory chain: a megacomplex organization?
Arch Biochem Biophys: 2013, 537(1);153-60
[PubMed:23880299]
[WorldCat.org]
[DOI]
(I p)
Gregory T Smaldone, Olga Revelles, Ahmed Gaballa, Uwe Sauer, Haike Antelmann, John D Helmann
A global investigation of the Bacillus subtilis iron-sparing response identifies major changes in metabolism.
J Bacteriol: 2012, 194(10);2594-605
[PubMed:22389480]
[WorldCat.org]
[DOI]
(I p)
Michael Baureder, Lars Hederstedt
Production, purification and detergent exchange of isotopically labeled Bacillussubtilis cytochrome b₅₅₈ (SdhC).
Protein Expr Purif: 2011, 80(1);97-101
[PubMed:21641999]
[WorldCat.org]
[DOI]
(I p)
Hannes Hahne, Susanne Wolff, Michael Hecker, Dörte Becher
From complementarity to comprehensiveness--targeting the membrane proteome of growing Bacillus subtilis by divergent approaches.
Proteomics: 2008, 8(19);4123-36
[PubMed:18763711]
[WorldCat.org]
[DOI]
(I p)
Ahmed Gaballa, Haike Antelmann, Claudio Aguilar, Sukhjit K Khakh, Kyung-Bok Song, Gregory T Smaldone, John D Helmann
The Bacillus subtilis iron-sparing response is mediated by a Fur-regulated small RNA and three small, basic proteins.
Proc Natl Acad Sci U S A: 2008, 105(33);11927-32
[PubMed:18697947]
[WorldCat.org]
[DOI]
(I p)
Victoria Yankovskaya, Rob Horsefield, Susanna Törnroth, César Luna-Chavez, Hideto Miyoshi, Christophe Léger, Bernadette Byrne, Gary Cecchini, So Iwata
Architecture of succinate dehydrogenase and reactive oxygen species generation.
Science: 2003, 299(5607);700-4
[PubMed:12560550]
[WorldCat.org]
[DOI]
(I p)
M Matsson, D Tolstoy, R Aasa, L Hederstedt
The distal heme center in Bacillus subtilis succinate:quinone reductase is crucial for electron transfer to menaquinone.
Biochemistry: 2000, 39(29);8617-24
[PubMed:10913269]
[WorldCat.org]
[DOI]
(P p)
C Hägerhäll, H Fridén, R Aasa, L Hederstedt
Transmembrane topology and axial ligands to hemes in the cytochrome b subunit of Bacillus subtilis succinate:menaquinone reductase.
Biochemistry: 1995, 34(35);11080-9
[PubMed:7669765]
[WorldCat.org]
[DOI]
(P p)
C Hägerhäll, R Aasa, C von Wachenfeldt, L Hederstedt
Two hemes in Bacillus subtilis succinate:menaquinone oxidoreductase (complex II).
Biochemistry: 1992, 31(32);7411-21
[PubMed:1324713]
[WorldCat.org]
[DOI]
(P p)
H Fridén, M R Cheesman, L Hederstedt, K K Andersson, A J Thomson
Low temperature EPR and MCD studies on cytochrome b-558 of the Bacillus subtilis succinate: quinone oxidoreductase indicate bis-histidine coordination of the heme iron.
Biochim Biophys Acta: 1990, 1041(2);207-15
[PubMed:2176107]
[WorldCat.org]
[DOI]
(P p)
L Melin, H Fridén, E Dehlin, L Rutberg, A von Gabain
The importance of the 5'-region in regulating the stability of sdh mRNA in Bacillus subtilis.
Mol Microbiol: 1990, 4(11);1881-9
[PubMed:1707123]
[WorldCat.org]
[DOI]
(P p)
H Fridén, L Hederstedt
Role of His residues in Bacillus subtilis cytochrome b558 for haem binding and assembly of succinate: quinone oxidoreductase (complex II).
Mol Microbiol: 1990, 4(6);1045-56
[PubMed:2120540]
[WorldCat.org]
[DOI]
(P p)
L Melin, L Rutberg, A von Gabain
Transcriptional and posttranscriptional control of the Bacillus subtilis succinate dehydrogenase operon.
J Bacteriol: 1989, 171(4);2110-5
[PubMed:2495271]
[WorldCat.org]
[DOI]
(P p)
H Fridén, L Rutberg, K Magnusson, L Hederstedt
Genetic and biochemical characterization of Bacillus subtilis mutants defective in expression and function of cytochrome b-558.
Eur J Biochem: 1987, 168(3);695-701
[PubMed:3117551]
[WorldCat.org]
[DOI]
(P p)
L Melin, K Magnusson, L Rutberg
Identification of the promoter of the Bacillus subtilis sdh operon.
J Bacteriol: 1987, 169(7);3232-6
[PubMed:3036777]
[WorldCat.org]
[DOI]
(P p)
S T Cole, C Condon, B D Lemire, J H Weiner
Molecular biology, biochemistry and bioenergetics of fumarate reductase, a complex membrane-bound iron-sulfur flavoenzyme of Escherichia coli.
Biochim Biophys Acta: 1985, 811(4);381-403
[PubMed:3910107]
[WorldCat.org]
[DOI]
(P p)
L Hederstedt, L Rutberg
Orientation of succinate dehydrogenase and cytochrome b558 in the Bacillus subtilis cytoplasmic membrane.
J Bacteriol: 1983, 153(1);57-65
[PubMed:6401289]
[WorldCat.org]
[DOI]
(P p)
L Hederstedt, L Rutberg
Succinate dehydrogenase--a comparative review.
Microbiol Rev: 1981, 45(4);542-55
[PubMed:6799760]
[WorldCat.org]
[DOI]
(P p)