Difference between revisions of "Sandbox"
| Line 1: | Line 1: | ||
| − | * '''Description:''' | + | * '''Description:''' similar to H+/glutamate symporter <br/><br/> |
{| align="right" border="1" cellpadding="2" | {| align="right" border="1" cellpadding="2" | ||
|- | |- | ||
|style="background:#ABCDEF;" align="center"|'''Gene name''' | |style="background:#ABCDEF;" align="center"|'''Gene name''' | ||
| − | |'' | + | |''gltP'' |
|- | |- | ||
|style="background:#ABCDEF;" align="center"| '''Synonyms''' || '' '' | |style="background:#ABCDEF;" align="center"| '''Synonyms''' || '' '' | ||
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''Essential''' || no | + | |style="background:#ABCDEF;" align="center"| '''Essential''' || no |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''Product''' || | + | |style="background:#ABCDEF;" align="center"| '''Product''' || unknown |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"|'''Function''' || | + | |style="background:#ABCDEF;" align="center"|'''Function''' || unknown |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''MW, pI''' || | + | |style="background:#ABCDEF;" align="center"| '''MW, pI''' || 44 kDa, 8.651 |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''Gene length, protein length''' || | + | |style="background:#ABCDEF;" align="center"| '''Gene length, protein length''' || 1242 bp, 414 aa |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[ | + | |style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[ybfQ]]'', ''[[gamP]]'' |
|- | |- | ||
| − | |colspan="2" style="background:#FAF8CC;" align="center"|'''Get the DNA and protein [http://srs.ebi.ac.uk/srsbin/cgi-bin/wgetz?-e+[EMBLCDS: | + | |colspan="2" style="background:#FAF8CC;" align="center"|'''Get the DNA and protein [http://srs.ebi.ac.uk/srsbin/cgi-bin/wgetz?-e+[EMBLCDS:CAB12028]+-newId sequences] <br/> (Barbe ''et al.'', 2009)''' |
|- | |- | ||
|- | |- | ||
|- | |- | ||
| − | |colspan="2" | '''Genetic context''' <br/> [[Image: | + | |colspan="2" | '''Genetic context''' <br/> [[Image:gltP_context.gif]] |
<div align="right"> <small>This image was kindly provided by [http://genolist.pasteur.fr/SubtiList/ SubtiList]</small></div> | <div align="right"> <small>This image was kindly provided by [http://genolist.pasteur.fr/SubtiList/ SubtiList]</small></div> | ||
|- | |- | ||
| Line 32: | Line 32: | ||
<br/><br/> | <br/><br/> | ||
| − | |||
=The gene= | =The gene= | ||
| Line 38: | Line 37: | ||
=== Basic information === | === Basic information === | ||
| − | * '''Locus tag:''' | + | * '''Locus tag:''' BSU02340 |
===Phenotypes of a mutant === | ===Phenotypes of a mutant === | ||
| − | |||
| − | |||
=== Database entries === | === Database entries === | ||
| − | * '''DBTBS entry:''' | + | * '''DBTBS entry:''' no entry |
| − | * '''SubtiList entry:''' [http://genolist.pasteur.fr/SubtiList/genome.cgi?gene_detail+ | + | * '''SubtiList entry:''' [http://genolist.pasteur.fr/SubtiList/genome.cgi?gene_detail+BG11015] |
=== Additional information=== | === Additional information=== | ||
| Line 57: | Line 54: | ||
=== Basic information/ Evolution === | === Basic information/ Evolution === | ||
| − | * '''Catalyzed reaction/ biological activity:''' | + | * '''Catalyzed reaction/ biological activity:''' |
| − | * '''Protein family:''' | + | * '''Protein family:''' View classification (according to Swiss-Prot) |
| − | * '''Paralogous protein(s):''' [[ | + | * '''Paralogous protein(s):''' [[GltT]], [[DctP]] |
=== Extended information on the protein === | === Extended information on the protein === | ||
| Line 68: | Line 65: | ||
* '''Domains:''' | * '''Domains:''' | ||
| − | |||
| − | |||
* '''Modification:''' | * '''Modification:''' | ||
| − | * '''Cofactor(s):''' | + | * '''Cofactor(s):''' |
* '''Effectors of protein activity:''' | * '''Effectors of protein activity:''' | ||
| Line 79: | Line 74: | ||
* '''Interactions:''' | * '''Interactions:''' | ||
| − | * '''Localization:''' membrane | + | * '''Localization:''' cell membrane (according to Swiss-Prot) |
=== Database entries === | === Database entries === | ||
| Line 85: | Line 80: | ||
* '''Structure:''' | * '''Structure:''' | ||
| − | * '''Swiss prot entry:''' [http://www.uniprot.org/uniprot/ | + | * '''Swiss prot entry:''' [http://www.uniprot.org/uniprot/P39817 P39817] |
| − | * '''KEGG entry:''' [http://www.genome.jp/dbget-bin/www_bget?bsu+ | + | * '''KEGG entry:''' [http://www.genome.jp/dbget-bin/www_bget?bsu+BSU02340] |
| − | * '''E.C. number:''' | + | * '''E.C. number:''' |
=== Additional information=== | === Additional information=== | ||
| − | |||
| − | |||
=Expression and regulation= | =Expression and regulation= | ||
| − | * '''Operon:' | + | * '''Operon:''' |
| − | * '''[[Sigma factor]]:''' | + | * '''[[Sigma factor]]:''' |
| − | * '''Regulation:''' | + | * '''Regulation:''' |
| − | * '''Regulatory mechanism:''' | + | * '''Regulatory mechanism:''' |
| − | * '''Additional information:''' | + | * '''Additional information:''' |
=Biological materials = | =Biological materials = | ||
| − | * '''Mutant:''' | + | * '''Mutant:''' |
* '''Expression vector:''' | * '''Expression vector:''' | ||
| − | + | ||
| − | * '''lacZ fusion:''' | + | * '''lacZ fusion:''' |
* '''GFP fusion:''' | * '''GFP fusion:''' | ||
| + | |||
| + | * '''two-hybrid system:''' | ||
* '''Antibody:''' | * '''Antibody:''' | ||
=Labs working on this gene/protein= | =Labs working on this gene/protein= | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
=Your additional remarks= | =Your additional remarks= | ||
| Line 133: | Line 120: | ||
=References= | =References= | ||
| − | <pubmed> | + | <pubmed>7751298, </pubmed> |
| − | # | + | # Tolner, B., Ubbink-Kok, T., Poolman, B. and Konings, W.N. 1995. Characterization of the proton/glutamate symport protein of Bacillus subtilis and its functional expression in Escherichia coli. J. Bacteriol. 177: 2863-2869. [http://www.ncbi.nlm.nih.gov/sites/entrez/7751298 PubMed] |
| − | + | # Author1, Author2 & Author3 (year) Title ''Journal'' '''volume:''' page-page. [http://www.ncbi.nlm.nih.gov/sites/entrez/PMID PubMed] | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | # | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
Revision as of 14:05, 8 June 2009
- Description: similar to H+/glutamate symporter
| Gene name | gltP |
| Synonyms | |
| Essential | no |
| Product | unknown |
| Function | unknown |
| MW, pI | 44 kDa, 8.651 |
| Gene length, protein length | 1242 bp, 414 aa |
| Immediate neighbours | ybfQ, gamP |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 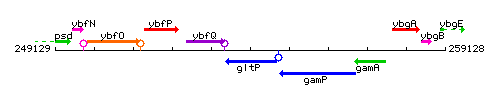
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU02340
Phenotypes of a mutant
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: View classification (according to Swiss-Prot)
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Interactions:
- Localization: cell membrane (according to Swiss-Prot)
Database entries
- Structure:
- Swiss prot entry: P39817
- KEGG entry: [2]
- E.C. number:
Additional information
Expression and regulation
- Operon:
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
B Tolner, T Ubbink-Kok, B Poolman, W N Konings
Characterization of the proton/glutamate symport protein of Bacillus subtilis and its functional expression in Escherichia coli.
J Bacteriol: 1995, 177(10);2863-9
[PubMed:7751298]
[WorldCat.org]
[DOI]
(P p)
- Tolner, B., Ubbink-Kok, T., Poolman, B. and Konings, W.N. 1995. Characterization of the proton/glutamate symport protein of Bacillus subtilis and its functional expression in Escherichia coli. J. Bacteriol. 177: 2863-2869. PubMed
- Author1, Author2 & Author3 (year) Title Journal volume: page-page. PubMed