Difference between revisions of "Sandbox"
| Line 1: | Line 1: | ||
| − | * '''Description:''' | + | * '''Description:''' DNA polymerase III (beta subunit), beta clamp, part of the [[replisome]], also involved in DNA mismatch repair, inhibitor of [[DnaA]] oligomerization <br/><br/> |
| − | |||
{| align="right" border="1" cellpadding="2" | {| align="right" border="1" cellpadding="2" | ||
|- | |- | ||
|style="background:#ABCDEF;" align="center"|'''Gene name''' | |style="background:#ABCDEF;" align="center"|'''Gene name''' | ||
| − | |'' | + | |''dnaN'' |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''Synonyms''' || '' | + | |style="background:#ABCDEF;" align="center"| '''Synonyms''' || ''dnaG, dnaK '' |
|- | |- | ||
|style="background:#ABCDEF;" align="center"| '''Essential''' || yes [http://www.ncbi.nlm.nih.gov/pubmed/12682299 PubMed] | |style="background:#ABCDEF;" align="center"| '''Essential''' || yes [http://www.ncbi.nlm.nih.gov/pubmed/12682299 PubMed] | ||
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''Product''' || | + | |style="background:#ABCDEF;" align="center"| '''Product''' || DNA polymerase III <br/>(beta subunit), beta clamp |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"|'''Function''' || [[DNA replication]] | + | |style="background:#ABCDEF;" align="center"|'''Function''' || [[DNA replication]], DNA repair |
|- | |- | ||
| − | |colspan="2" style="background:#FAF8CC;" align="center"| '''Gene expression levels in [http://subtiwiki.uni-goettingen.de/apps/expression/ ''Subti''Express]''': [http://subtiwiki.uni-goettingen.de/apps/expression/expression.php?search= | + | |colspan="2" style="background:#FAF8CC;" align="center"| '''Gene expression levels in [http://subtiwiki.uni-goettingen.de/apps/expression/ ''Subti''Express]''': [http://subtiwiki.uni-goettingen.de/apps/expression/expression.php?search=BSU00020 DnaN] |
|- | |- | ||
| − | |colspan="2" style="background:#FAF8CC;" align="center"| '''Interactions involving this protein in [http://subtiwiki.uni-goettingen.de/interact/ ''Subt''Interact]''': [http://subtiwiki.uni-goettingen.de/interact/index.php?protein= | + | |colspan="2" style="background:#FAF8CC;" align="center"| '''Interactions involving this protein in [http://subtiwiki.uni-goettingen.de/interact/ ''Subt''Interact]''': [http://subtiwiki.uni-goettingen.de/interact/index.php?protein=DnaN DnaN] |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''MW, pI''' || | + | |style="background:#ABCDEF;" align="center"| '''MW, pI''' || 41 kDa, 4.718 |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''Gene length, protein length''' || | + | |style="background:#ABCDEF;" align="center"| '''Gene length, protein length''' || 1134 bp, 378 aa |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[ | + | |style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[dnaA]]'', ''[[yaaA]]'' |
|- | |- | ||
| − | |style="background:#FAF8CC;" align="center"|'''Sequences'''||[http://bsubcyc.org/BSUB/sequence-aa?type=GENE&object= | + | |style="background:#FAF8CC;" align="center"|'''Sequences'''||[http://bsubcyc.org/BSUB/sequence-aa?type=GENE&object=BSU00020 Protein] [http://bsubcyc.org/BSUB/sequence?type=GENE&object=BSU00020 DNA] [http://bsubcyc.org/BSUB/seq-selector?chromosome=CHROM-1&object=BSU00020 DNA_with_flanks] |
|- | |- | ||
|colspan="2" | '''Genetic context''' <br/> [[Image:DnaA_dnaN_yaaA_recF_yaaB_gyrB_context.png]] | |colspan="2" | '''Genetic context''' <br/> [[Image:DnaA_dnaN_yaaA_recF_yaaB_gyrB_context.png]] | ||
<div align="right"> <small>This image was kindly provided by [http://genolist.pasteur.fr/SubtiList/ SubtiList]</small></div> | <div align="right"> <small>This image was kindly provided by [http://genolist.pasteur.fr/SubtiList/ SubtiList]</small></div> | ||
|- | |- | ||
| − | |colspan="2" |'''[http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id= | + | |colspan="2" |'''[http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=dnaN_1939_3075_1 Expression at a glance]'''   {{PubMed|22383849}}<br/>[[Image:DnaN_expression.png|500px|link=http://subtiwiki.uni-goettingen.de/apps/expression/expression.php?search=BSU00020]] |
|- | |- | ||
|} | |} | ||
| Line 45: | Line 44: | ||
= This gene is a member of the following [[regulons]] = | = This gene is a member of the following [[regulons]] = | ||
{{SubtiWiki regulon|[[Spo0A regulon]]}} | {{SubtiWiki regulon|[[Spo0A regulon]]}} | ||
| − | + | ||
=The gene= | =The gene= | ||
=== Basic information === | === Basic information === | ||
| − | * '''Locus tag:''' | + | * '''Locus tag:''' BSU00020 |
===Phenotypes of a mutant === | ===Phenotypes of a mutant === | ||
| Line 60: | Line 59: | ||
* '''DBTBS entry:''' [http://dbtbs.hgc.jp/COG/prom/dnaAN.html] | * '''DBTBS entry:''' [http://dbtbs.hgc.jp/COG/prom/dnaAN.html] | ||
| − | * '''SubtiList entry:''' [http://genolist.pasteur.fr/SubtiList/genome.cgi?gene_detail+ | + | * '''SubtiList entry:''' [http://genolist.pasteur.fr/SubtiList/genome.cgi?gene_detail+BG10066] |
=== Additional information=== | === Additional information=== | ||
| − | |||
=The protein= | =The protein= | ||
| Line 70: | Line 68: | ||
* '''Catalyzed reaction/ biological activity:''' | * '''Catalyzed reaction/ biological activity:''' | ||
| − | ** | + | ** [[DnaN]] overexpression or release from the [[replisome]] decreases association of [[YabA]] with ''oriC'', increases association of [[DnaA]] with ''oriC'' {{PubMed|21895792}} |
| + | ** Deoxynucleoside triphosphate + DNA(n) = diphosphate + DNA(n+1) (according to Swiss-Prot) | ||
| + | ** recruits [[MutS]] to the site of [[DNA replication]] ([[replisome]]) {{PubMed|23228104}} | ||
| + | ** required for bacteriophage SPP1 replication {{PubMed|23268446}} | ||
| + | ** inhibits oligomerization and helix formation of [[DnaA]] {{PubMed|23909787}} | ||
| − | * '''Protein family:''' | + | * '''Protein family:''' |
* '''Paralogous protein(s):''' | * '''Paralogous protein(s):''' | ||
| Line 80: | Line 82: | ||
* '''Kinetic information:''' | * '''Kinetic information:''' | ||
| − | * '''Domains:''' | + | * '''Domains:''' |
* '''Modification:''' | * '''Modification:''' | ||
| Line 87: | Line 89: | ||
* '''Effectors of protein activity:''' | * '''Effectors of protein activity:''' | ||
| − | |||
| − | |||
| − | |||
| − | |||
* '''[[SubtInteract|Interactions]]:''' | * '''[[SubtInteract|Interactions]]:''' | ||
| − | ** [[DnaA]] | + | ** [[DnaA]]-[[YabA]]-[[DnaN]] {{PubMed|12060778}} |
| − | ** [[ | + | ** part of the [[replisome]]: [[PolC]]-[[HolA]]-[[HolB]]-[[DnaX]]-[[DnaN]]-[[DnaG]]-[[DnaC]]-[[DnaI]]-[[DnaD]]-[[SsbA]]-[[DnaE]]-[[PriA]]-[[DnaB]] {{PubMed|20122408}} |
| − | ** [[ | + | ** [[PolY1]]-[[DnaN]], [[PolY2]]-[[DnaN]] (required for untargeted mutagenesis) {{PubMed|15469515}} |
| − | ** [[ | + | ** [[PolY1]]-[[PolA]]-[[DnaN]] {{PubMed|16045613}} |
| − | ** [[ | + | ** [[MutL]] (C-terminal domain)-[[DnaN]] {{PubMed|21050827}} |
| − | ** [[ | + | ** [[MutS]]-[[DnaN]] {{PubMed|20453097}} |
| − | ** [[ | + | ** [[DnaE]]-[[DnaN]] {{PubMed|21958350}} |
| − | ** | + | ** [[DnaN]] forms dimers {{PubMed|16045613}} |
* '''[[Localization]]:''' | * '''[[Localization]]:''' | ||
| − | ** | + | ** nucleoid (mid-cell spot) [http://www.ncbi.nlm.nih.gov/sites/entrez/9822387 PubMed] [http://www.ncbi.nlm.nih.gov/sites/entrez/16479537 PubMed] |
| + | ** forms large assemblies on DNA, called "clamp zones" {{PubMed|21419346}} | ||
| + | ** forms foci {{PubMed|21958350}} | ||
=== Database entries === | === Database entries === | ||
| Line 109: | Line 109: | ||
* '''Structure:''' | * '''Structure:''' | ||
| − | * '''UniProt:''' [http://www.uniprot.org/uniprot/ | + | * '''UniProt:''' [http://www.uniprot.org/uniprot/P05649 P05649] |
| − | * '''KEGG entry:''' [http://www.genome.jp/dbget-bin/www_bget?bsu: | + | * '''KEGG entry:''' [http://www.genome.jp/dbget-bin/www_bget?bsu:BSU00020] |
| − | * '''E.C. number:''' | + | * '''E.C. number:''' [http://www.expasy.org/enzyme/2.7.7.7 2.7.7.7] |
=== Additional information=== | === Additional information=== | ||
| Line 121: | Line 121: | ||
* '''Operon:''' ''[[dnaA]]-[[dnaN]]'' [http://www.ncbi.nlm.nih.gov/sites/entrez/2987848 PubMed] | * '''Operon:''' ''[[dnaA]]-[[dnaN]]'' [http://www.ncbi.nlm.nih.gov/sites/entrez/2987848 PubMed] | ||
| − | * '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id= | + | * '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=dnaN_1939_3075_1 dnaN] {{PubMed|22383849}} |
| − | * '''[[Sigma factor]]:''' [[SigA]] | + | * '''[[Sigma factor]]:''' [[SigA]] {{PubMed|2987848}} |
| − | * '''Regulation:''' | + | * '''Regulation:''' negatively controlled by [[DnaA]] [http://www.ncbi.nlm.nih.gov/sites/entrez/2168872 PubMed] and [[Spo0A]] [http://www.ncbi.nlm.nih.gov/sites/entrez/14651647 PubMed] |
| − | |||
** repressed under conditions that trigger sporulation ([[Spo0A]]) [http://www.ncbi.nlm.nih.gov/sites/entrez/14651647 PubMed] | ** repressed under conditions that trigger sporulation ([[Spo0A]]) [http://www.ncbi.nlm.nih.gov/sites/entrez/14651647 PubMed] | ||
| Line 142: | Line 141: | ||
* '''lacZ fusion:''' | * '''lacZ fusion:''' | ||
| − | * '''GFP fusion:''' | + | * '''GFP fusion:''' [http://www.ncbi.nlm.nih.gov/sites/entrez/16942601 PubMed] [http://www.ncbi.nlm.nih.gov/sites/entrez/9822387 PubMed] |
* '''two-hybrid system:''' | * '''two-hybrid system:''' | ||
| Line 149: | Line 148: | ||
=Labs working on this gene/protein= | =Labs working on this gene/protein= | ||
| − | + | [[Philippe Noirot]], Jouy-en-Josas, France [http://locus.jouy.inra.fr/cms/index.php?id=18 homepage] | |
| − | |||
| − | |||
| − | |||
=Your additional remarks= | =Your additional remarks= | ||
| Line 158: | Line 154: | ||
=References= | =References= | ||
==Reviews== | ==Reviews== | ||
| − | <pubmed> 20157337 | + | <pubmed> 20157337 22933559 </pubmed> |
| − | + | == Original publications == | |
| − | + | <pubmed>16942601 9822387 14651647,2987848 2168872 14651647 12060778 16461910, 16479537 19737352 19081080 11395445 20122408 20451384 15469515 16045613 21895792 20453097 23228104 23268446,21050827,21419346,21958350 23909787 </pubmed> | |
| − | |||
| − | ==Original publications== | ||
| − | <pubmed> | ||
| − | |||
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 14:26, 11 November 2013
- Description: DNA polymerase III (beta subunit), beta clamp, part of the replisome, also involved in DNA mismatch repair, inhibitor of DnaA oligomerization
| Gene name | dnaN |
| Synonyms | dnaG, dnaK |
| Essential | yes PubMed |
| Product | DNA polymerase III (beta subunit), beta clamp |
| Function | DNA replication, DNA repair |
| Gene expression levels in SubtiExpress: DnaN | |
| Interactions involving this protein in SubtInteract: DnaN | |
| MW, pI | 41 kDa, 4.718 |
| Gene length, protein length | 1134 bp, 378 aa |
| Immediate neighbours | dnaA, yaaA |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed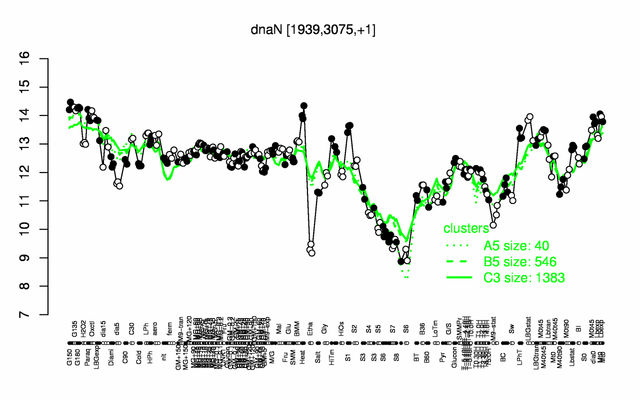
| |
Contents
Categories containing this gene/protein
DNA replication, essential genes
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU00020
Phenotypes of a mutant
essential PubMed
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- DnaN overexpression or release from the replisome decreases association of YabA with oriC, increases association of DnaA with oriC PubMed
- Deoxynucleoside triphosphate + DNA(n) = diphosphate + DNA(n+1) (according to Swiss-Prot)
- recruits MutS to the site of DNA replication (replisome) PubMed
- required for bacteriophage SPP1 replication PubMed
- inhibits oligomerization and helix formation of DnaA PubMed
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Interactions:
- DnaA-YabA-DnaN PubMed
- part of the replisome: PolC-HolA-HolB-DnaX-DnaN-DnaG-DnaC-DnaI-DnaD-SsbA-DnaE-PriA-DnaB PubMed
- PolY1-DnaN, PolY2-DnaN (required for untargeted mutagenesis) PubMed
- PolY1-PolA-DnaN PubMed
- MutL (C-terminal domain)-DnaN PubMed
- MutS-DnaN PubMed
- DnaE-DnaN PubMed
- DnaN forms dimers PubMed
Database entries
- Structure:
- UniProt: P05649
- KEGG entry: [3]
- E.C. number: 2.7.7.7
Additional information
Expression and regulation
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Philippe Noirot, Jouy-en-Josas, France homepage
Your additional remarks
References
Reviews
Justin S Lenhart, Jeremy W Schroeder, Brian W Walsh, Lyle A Simmons
DNA repair and genome maintenance in Bacillus subtilis.
Microbiol Mol Biol Rev: 2012, 76(3);530-64
[PubMed:22933559]
[WorldCat.org]
[DOI]
(I p)
Tsutomu Katayama, Shogo Ozaki, Kenji Keyamura, Kazuyuki Fujimitsu
Regulation of the replication cycle: conserved and diverse regulatory systems for DnaA and oriC.
Nat Rev Microbiol: 2010, 8(3);163-70
[PubMed:20157337]
[WorldCat.org]
[DOI]
(I p)
Original publications
Graham Scholefield, Heath Murray
YabA and DnaD inhibit helix assembly of the DNA replication initiation protein DnaA.
Mol Microbiol: 2013, 90(1);147-59
[PubMed:23909787]
[WorldCat.org]
[DOI]
(I p)
Elena M Seco, John C Zinder, Carol M Manhart, Ambra Lo Piano, Charles S McHenry, Silvia Ayora
Bacteriophage SPP1 DNA replication strategies promote viral and disable host replication in vitro.
Nucleic Acids Res: 2013, 41(3);1711-21
[PubMed:23268446]
[WorldCat.org]
[DOI]
(I p)
Justin S Lenhart, Anushi Sharma, Manju M Hingorani, Lyle A Simmons
DnaN clamp zones provide a platform for spatiotemporal coupling of mismatch detection to DNA replication.
Mol Microbiol: 2013, 87(3);553-68
[PubMed:23228104]
[WorldCat.org]
[DOI]
(I p)
Andrew D Klocko, Jeremy W Schroeder, Brian W Walsh, Justin S Lenhart, Margery L Evans, Lyle A Simmons
Mismatch repair causes the dynamic release of an essential DNA polymerase from the replication fork.
Mol Microbiol: 2011, 82(3);648-63
[PubMed:21958350]
[WorldCat.org]
[DOI]
(I p)
Houra Merrikh, Alan D Grossman
Control of the replication initiator DnaA by an anti-cooperativity factor.
Mol Microbiol: 2011, 82(2);434-46
[PubMed:21895792]
[WorldCat.org]
[DOI]
(I p)
Masayuki Su'etsugu, Jeff Errington
The replicase sliding clamp dynamically accumulates behind progressing replication forks in Bacillus subtilis cells.
Mol Cell: 2011, 41(6);720-32
[PubMed:21419346]
[WorldCat.org]
[DOI]
(I p)
Monica C Pillon, Jeffrey H Miller, Alba Guarné
The endonuclease domain of MutL interacts with the β sliding clamp.
DNA Repair (Amst): 2011, 10(1);87-93
[PubMed:21050827]
[WorldCat.org]
[DOI]
(I p)
Nicole M Dupes, Brian W Walsh, Andrew D Klocko, Justin S Lenhart, Heather L Peterson, David A Gessert, Cassie E Pavlick, Lyle A Simmons
Mutations in the Bacillus subtilis beta clamp that separate its roles in DNA replication from mismatch repair.
J Bacteriol: 2010, 192(13);3452-63
[PubMed:20453097]
[WorldCat.org]
[DOI]
(I p)
Peter T McKenney, Adam Driks, Haig A Eskandarian, Paul Grabowski, Jonathan Guberman, Katherine H Wang, Zemer Gitai, Patrick Eichenberger
A distance-weighted interaction map reveals a previously uncharacterized layer of the Bacillus subtilis spore coat.
Curr Biol: 2010, 20(10);934-8
[PubMed:20451384]
[WorldCat.org]
[DOI]
(I p)
Glenn M Sanders, H Garry Dallmann, Charles S McHenry
Reconstitution of the B. subtilis replisome with 13 proteins including two distinct replicases.
Mol Cell: 2010, 37(2);273-81
[PubMed:20122408]
[WorldCat.org]
[DOI]
(I p)
Alexi I Goranov, Adam M Breier, Houra Merrikh, Alan D Grossman
YabA of Bacillus subtilis controls DnaA-mediated replication initiation but not the transcriptional response to replication stress.
Mol Microbiol: 2009, 74(2);454-66
[PubMed:19737352]
[WorldCat.org]
[DOI]
(I p)
Clarisse Defeu Soufo, Hervé Joël Defeu Soufo, Marie-Françoise Noirot-Gros, Astrid Steindorf, Philippe Noirot, Peter L Graumann
Cell-cycle-dependent spatial sequestration of the DnaA replication initiator protein in Bacillus subtilis.
Dev Cell: 2008, 15(6);935-41
[PubMed:19081080]
[WorldCat.org]
[DOI]
(I p)
Melanie B Berkmen, Alan D Grossman
Spatial and temporal organization of the Bacillus subtilis replication cycle.
Mol Microbiol: 2006, 62(1);57-71
[PubMed:16942601]
[WorldCat.org]
[DOI]
(P p)
Jean-Christophe Meile, Ling Juan Wu, S Dusko Ehrlich, Jeff Errington, Philippe Noirot
Systematic localisation of proteins fused to the green fluorescent protein in Bacillus subtilis: identification of new proteins at the DNA replication factory.
Proteomics: 2006, 6(7);2135-46
[PubMed:16479537]
[WorldCat.org]
[DOI]
(P p)
Marie-Françoise Noirot-Gros, M Velten, M Yoshimura, S McGovern, T Morimoto, S D Ehrlich, N Ogasawara, P Polard, Philippe Noirot
Functional dissection of YabA, a negative regulator of DNA replication initiation in Bacillus subtilis.
Proc Natl Acad Sci U S A: 2006, 103(7);2368-73
[PubMed:16461910]
[WorldCat.org]
[DOI]
(P p)
Stéphane Duigou, S Dusko Ehrlich, Philippe Noirot, Marie-Françoise Noirot-Gros
DNA polymerase I acts in translesion synthesis mediated by the Y-polymerases in Bacillus subtilis.
Mol Microbiol: 2005, 57(3);678-90
[PubMed:16045613]
[WorldCat.org]
[DOI]
(P p)
Stéphane Duigou, S Dusko Ehrlich, Philippe Noirot, Marie-Françoise Noirot-Gros
Distinctive genetic features exhibited by the Y-family DNA polymerases in Bacillus subtilis.
Mol Microbiol: 2004, 54(2);439-51
[PubMed:15469515]
[WorldCat.org]
[DOI]
(P p)
Virginie Molle, Masaya Fujita, Shane T Jensen, Patrick Eichenberger, José E González-Pastor, Jun S Liu, Richard Losick
The Spo0A regulon of Bacillus subtilis.
Mol Microbiol: 2003, 50(5);1683-701
[PubMed:14651647]
[WorldCat.org]
[DOI]
(P p)
Marie-Françoise Noirot-Gros, Etienne Dervyn, Ling Juan Wu, Peggy Mervelet, Jeffery Errington, S Dusko Ehrlich, Philippe Noirot
An expanded view of bacterial DNA replication.
Proc Natl Acad Sci U S A: 2002, 99(12);8342-7
[PubMed:12060778]
[WorldCat.org]
[DOI]
(P p)
Y Ogura, Y Imai, N Ogasawara, S Moriya
Autoregulation of the dnaA-dnaN operon and effects of DnaA protein levels on replication initiation in Bacillus subtilis.
J Bacteriol: 2001, 183(13);3833-41
[PubMed:11395445]
[WorldCat.org]
[DOI]
(P p)
K P Lemon, A D Grossman
Localization of bacterial DNA polymerase: evidence for a factory model of replication.
Science: 1998, 282(5393);1516-9
[PubMed:9822387]
[WorldCat.org]
[DOI]
(P p)
T Fukuoka, S Moriya, H Yoshikawa, N Ogasawara
Purification and characterization of an initiation protein for chromosomal replication, DnaA, in Bacillus subtilis.
J Biochem: 1990, 107(5);732-9
[PubMed:2168872]
[WorldCat.org]
[DOI]
(P p)
N Ogasawara, S Moriya, H Yoshikawa
Structure and function of the region of the replication origin of the Bacillus subtilis chromosome. IV. Transcription of the oriC region and expression of DNA gyrase genes and other open reading frames.
Nucleic Acids Res: 1985, 13(7);2267-79
[PubMed:2987848]
[WorldCat.org]
[DOI]
(P p)