Difference between revisions of "Sandbox"
| Line 1: | Line 1: | ||
| − | * '''Description:''' | + | * '''Description:''' write here <br/><br/> |
{| align="right" border="1" cellpadding="2" | {| align="right" border="1" cellpadding="2" | ||
|- | |- | ||
|style="background:#ABCDEF;" align="center"|'''Gene name''' | |style="background:#ABCDEF;" align="center"|'''Gene name''' | ||
| − | |'' | + | |''yaaB'' |
|- | |- | ||
|style="background:#ABCDEF;" align="center"| '''Synonyms''' || '' '' | |style="background:#ABCDEF;" align="center"| '''Synonyms''' || '' '' | ||
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''Essential''' || | + | |style="background:#ABCDEF;" align="center"| '''Essential''' || no |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''Product''' || | + | |style="background:#ABCDEF;" align="center"| '''Product''' || |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"|'''Function''' || | + | |style="background:#ABCDEF;" align="center"|'''Function''' || unknown |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''MW, pI''' || | + | |style="background:#ABCDEF;" align="center"| '''MW, pI''' || 5 kDa, 8.796 |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''Gene length, protein length''' || | + | |style="background:#ABCDEF;" align="center"| '''Gene length, protein length''' || 156 bp, 52 aa |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || | + | |style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || |
|- | |- | ||
| − | |style="background:#FAF8CC;" align="center"|'''[http://subtiwiki.uni-goettingen.de/ | + | |style="background:#FAF8CC;" align="center"|'''[http://subtiwiki.uni-goettingen.de/yaaB_nucleotide.txt Gene sequence (+200bp) ]''' |
| − | |style="background:#FAF8CC;" align="center"|'''[http://subtiwiki.uni-goettingen.de/ | + | |style="background:#FAF8CC;" align="center"|'''[http://subtiwiki.uni-goettingen.de/yaaB_protein.txt Protein sequence]''' |
|- | |- | ||
| − | |colspan="2" | '''Genetic context''' <br/> [[Image: | + | |colspan="2" | '''Genetic context''' <br/> [[Image:yaaB_context.gif]] |
|- | |- | ||
|} | |} | ||
| Line 31: | Line 31: | ||
<br/><br/> | <br/><br/> | ||
| − | |||
=The gene= | =The gene= | ||
=== Basic information === | === Basic information === | ||
| − | * '''Coordinates:''' | + | * '''Coordinates:''' |
===Phenotypes of a mutant === | ===Phenotypes of a mutant === | ||
| − | |||
| − | |||
=== Database entries === | === Database entries === | ||
| − | * '''DBTBS entry:''' [http://dbtbs.hgc.jp/COG/prom/ | + | * '''DBTBS entry:''' [http://dbtbs.hgc.jp/COG/prom/yaaA-recF-yaaB-gyrB.html] |
| − | * '''SubtiList entry:'''[http://genolist.pasteur.fr/SubtiList/genome.cgi?gene_detail+ | + | * '''SubtiList entry:''' [http://genolist.pasteur.fr/SubtiList/genome.cgi?gene_detail+BG10069] |
=== Additional information=== | === Additional information=== | ||
| + | |||
=The protein= | =The protein= | ||
| Line 54: | Line 52: | ||
=== Basic information/ Evolution === | === Basic information/ Evolution === | ||
| − | * '''Catalyzed reaction/ biological activity:''' | + | * '''Catalyzed reaction/ biological activity:''' |
* '''Protein family:''' | * '''Protein family:''' | ||
| − | * '''Paralogous protein(s):''' | + | * '''Paralogous protein(s):''' |
=== Extended information on the protein === | === Extended information on the protein === | ||
| − | * '''Kinetic information:''' | + | * '''Kinetic information:''' |
* '''Domains:''' | * '''Domains:''' | ||
| − | * '''Modification:''' | + | * '''Modification:''' |
* '''Cofactor(s):''' | * '''Cofactor(s):''' | ||
| Line 72: | Line 70: | ||
* '''Effectors of protein activity:''' | * '''Effectors of protein activity:''' | ||
| − | * '''Interactions:''' | + | * '''Interactions:''' |
| − | |||
| − | |||
| − | * '''Localization:''' | + | * '''Localization:''' |
=== Database entries === | === Database entries === | ||
| − | * '''Structure:''' | + | * '''Structure:''' |
| − | |||
| − | |||
| − | * '''Swiss prot entry:''' | + | * '''Swiss prot entry:''' |
| − | * '''KEGG entry:''' | + | * '''KEGG entry:''' |
| − | * '''E.C. number:''' | + | * '''E.C. number:''' |
=== Additional information=== | === Additional information=== | ||
| − | |||
| − | |||
=Expression and regulation= | =Expression and regulation= | ||
| − | * '''Operon:''' | + | * '''Operon:''' ''[[yaaA]]-[[recF]]-[[yaaB]]-[[gyrB]]'' [http://www.ncbi.nlm.nih.gov/sites/entrez/2987848 PubMed] |
| − | |||
| − | |||
| − | + | * '''Sigma factor:''' [[SigA]] [http://www.ncbi.nlm.nih.gov/sites/entrez/2987848 PubMed] | |
| − | * ''' | + | * '''Regulation:''' |
| − | * ''' | + | * '''Regulatory mechanism:''' |
| − | + | * '''Additional information:''' | |
| − | |||
| − | |||
| − | |||
| − | * '''Additional information:''' | ||
=Biological materials = | =Biological materials = | ||
| − | * '''Mutant:''' | + | * '''Mutant:''' |
| − | * '''Expression vector:''' | + | * '''Expression vector:''' |
| − | + | ||
| − | * '''lacZ fusion:''' | + | * '''lacZ fusion:''' |
* '''GFP fusion:''' | * '''GFP fusion:''' | ||
| − | * '''two-hybrid system:''' | + | * '''two-hybrid system:''' |
| − | * '''Antibody:''' | + | * '''Antibody:''' |
=Labs working on this gene/protein= | =Labs working on this gene/protein= | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
=Your additional remarks= | =Your additional remarks= | ||
| Line 137: | Line 118: | ||
=References= | =References= | ||
| − | # | + | # Ogasawara et al. (1985) Structure and function of the region of the replication origin of the ''Bacillus subtilis'' chromosome. IV. Transcription of the oriC region and expression of DNA gyrase genes and other open reading frames.''Nucl. Acids Res.'' '''11:''' 2267-2279. [http://www.ncbi.nlm.nih.gov/sites/entrez/2987848 PubMed] |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
Revision as of 16:23, 11 February 2009
- Description: write here
| Gene name | yaaB |
| Synonyms | |
| Essential | no |
| Product | |
| Function | unknown |
| MW, pI | 5 kDa, 8.796 |
| Gene length, protein length | 156 bp, 52 aa |
| Immediate neighbours | |
| Gene sequence (+200bp) | Protein sequence |
Genetic context 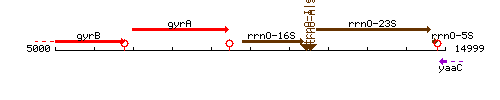
| |
Contents
The gene
Basic information
- Coordinates:
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Interactions:
- Localization:
Database entries
- Structure:
- Swiss prot entry:
- KEGG entry:
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
- Ogasawara et al. (1985) Structure and function of the region of the replication origin of the Bacillus subtilis chromosome. IV. Transcription of the oriC region and expression of DNA gyrase genes and other open reading frames.Nucl. Acids Res. 11: 2267-2279. PubMed