Difference between revisions of "RpsO"
(→References) |
|||
| Line 139: | Line 139: | ||
=References= | =References= | ||
| − | + | <pubmed> 19633085 19653700 20418391 10497132 11846555 23002217 16005889 23611891 </pubmed> | |
| − | <pubmed> 19633085 19653700 20418391 10497132</pubmed> | ||
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 10:46, 26 April 2013
- Description: ribosomal protein
| Gene name | rpsO |
| Synonyms | |
| Essential | yes PubMed |
| Product | ribosomal protein S15 (BS18) |
| Function | translation |
| Gene expression levels in SubtiExpress: rpsO | |
| Interactions involving this protein in SubtInteract: RpsO | |
| MW, pI | 10 kDa, 10.975 |
| Gene length, protein length | 267 bp, 89 aa |
| Immediate neighbours | ribC, pnpA |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 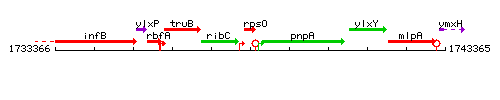
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed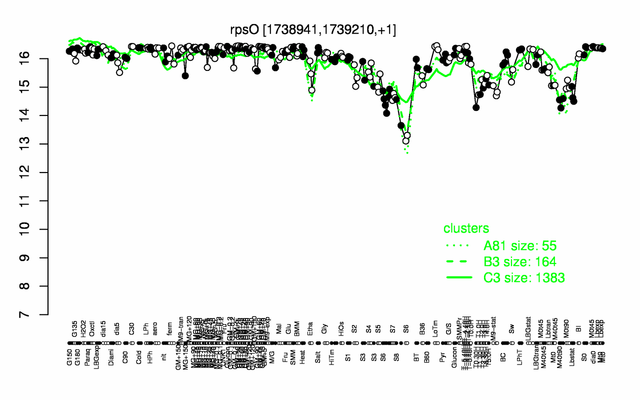
| |
Contents
Categories containing this gene/protein
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU16680
Phenotypes of a mutant
essential PubMed
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: ribosomal protein S15P family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- Structure: 1A32 (Geobacillus stearothermophilus)
- UniProt: P21473
- KEGG entry: [2]
- E.C. number:
Additional information
Expression and regulation
- Operon: rpsO PubMed
- Sigma factor:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Kaila Deiorio-Haggar, Jon Anthony, Michelle M Meyer
RNA structures regulating ribosomal protein biosynthesis in bacilli.
RNA Biol: 2013, 10(7);1180-4
[PubMed:23611891]
[WorldCat.org]
[DOI]
(I p)
Genki Akanuma, Hideaki Nanamiya, Yousuke Natori, Koichi Yano, Shota Suzuki, Shuya Omata, Morio Ishizuka, Yasuhiko Sekine, Fujio Kawamura
Inactivation of ribosomal protein genes in Bacillus subtilis reveals importance of each ribosomal protein for cell proliferation and cell differentiation.
J Bacteriol: 2012, 194(22);6282-91
[PubMed:23002217]
[WorldCat.org]
[DOI]
(I p)
Shiyi Yao, David H Bechhofer
Initiation of decay of Bacillus subtilis rpsO mRNA by endoribonuclease RNase Y.
J Bacteriol: 2010, 192(13);3279-86
[PubMed:20418391]
[WorldCat.org]
[DOI]
(I p)
Matthew A Lauber, William E Running, James P Reilly
B. subtilis ribosomal proteins: structural homology and post-translational modifications.
J Proteome Res: 2009, 8(9);4193-206
[PubMed:19653700]
[WorldCat.org]
[DOI]
(P p)
Shiyi Yao, David H Bechhofer
Processing and stability of inducibly expressed rpsO mRNA derivatives in Bacillus subtilis.
J Bacteriol: 2009, 191(18);5680-9
[PubMed:19633085]
[WorldCat.org]
[DOI]
(I p)
Lincoln G Scott, James R Williamson
The binding interface between Bacillus stearothermophilus ribosomal protein S15 and its 5'-translational operator mRNA.
J Mol Biol: 2005, 351(2);280-90
[PubMed:16005889]
[WorldCat.org]
[DOI]
(P p)
L G Scott, J R Williamson
Interaction of the Bacillus stearothermophilus ribosomal protein S15 with its 5'-translational operator mRNA.
J Mol Biol: 2001, 314(3);413-22
[PubMed:11846555]
[WorldCat.org]
[DOI]
(P p)
G M Culver, J H Cate, G Z Yusupova, M M Yusupov, H F Noller
Identification of an RNA-protein bridge spanning the ribosomal subunit interface.
Science: 1999, 285(5436);2133-6
[PubMed:10497132]
[WorldCat.org]
[DOI]
(P p)