Difference between revisions of "RpoA"
(→Original publications) |
|||
| Line 154: | Line 154: | ||
<pubmed> 22210308 </pubmed> | <pubmed> 22210308 </pubmed> | ||
==Original publications== | ==Original publications== | ||
| − | <pubmed>20084284 19726675 19580872 ,3093467,2496109,8635744,10675340,12486038,18643936, 12642660, 16249335, 16740936 15378759 22512862,20817769,22307755</pubmed> | + | <pubmed>20084284 19726675 19580872 ,3093467,2496109,8635744,10675340,12486038,18643936, 12642660, 16249335, 16740936 15378759 26154296 22512862,20817769,22307755</pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 14:00, 13 July 2015
- Description: RNA polymerase alpha subunit
| Gene name | rpoA |
| Synonyms | |
| Essential | yes PubMed |
| Product | RNA polymerase alpha subunit |
| Function | transcription |
| Gene expression levels in SubtiExpress: rpoA | |
| Interactions involving this protein in SubtInteract: RpoA | |
| MW, pI | 34 kDa, 4.593 |
| Gene length, protein length | 942 bp, 314 aa |
| Immediate neighbours | rpsK, rplQ |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 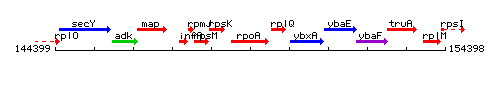
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed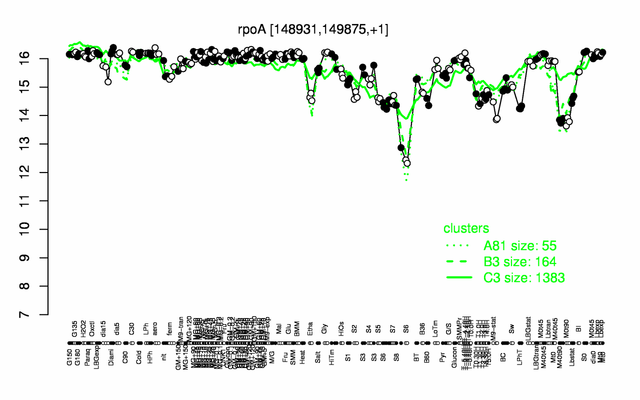
| |
Contents
Categories containing this gene/protein
transcription, essential genes, most abundant proteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU01430
Phenotypes of a mutant
essential PubMed
Database entries
- BsubCyc: BSU01430
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: Nucleoside triphosphate + RNA(n) = diphosphate + RNA(n+1) (according to Swiss-Prot)
- Protein family: RNA polymerase alpha chain family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Modification:
- Effectors of protein activity:
Database entries
- BsubCyc: BSU01430
- Structure: 1Z3E (C-terminal domain, complex with Spx)
- UniProt: P20429
- KEGG entry: [2]
- E.C. number: 2.7.7.6
Additional information
Expression and regulation
- Operon:
- Regulatory mechanism:
- Additional information:
- belongs to the 100 most abundant proteins PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium): 3504 PubMed
- number of protein molecules per cell (complex medium with amino acids, without glucose): 11832 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium, exponential phase): 27116 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium, early stationary phase after glucose exhaustion): 13650 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium, late stationary phase after glucose exhaustion): 25767 PubMed
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system: B. pertussis adenylate cyclase-based bacterial two hybrid system (BACTH), available in Stülke lab
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Lakshminarayan M Iyer, L Aravind
Insights from the architecture of the bacterial transcription apparatus.
J Struct Biol: 2012, 179(3);299-319
[PubMed:22210308]
[WorldCat.org]
[DOI]
(I p)
Original publications
Satohiko Murayama, Shu Ishikawa, Onuma Chumsakul, Naotake Ogasawara, Taku Oshima
The Role of α-CTD in the Genome-Wide Transcriptional Regulation of the Bacillus subtilis Cells.
PLoS One: 2015, 10(7);e0131588
[PubMed:26154296]
[WorldCat.org]
[DOI]
(I e)
Andrea Wünsche, Elke Hammer, Maike Bartholomae, Uwe Völker, Andreas Burkovski, Gerald Seidel, Wolfgang Hillen
CcpA forms complexes with CodY and RpoA in Bacillus subtilis.
FEBS J: 2012, 279(12);2201-14
[PubMed:22512862]
[WorldCat.org]
[DOI]
(I p)
Ann A Lin, Peter Zuber
Evidence that a single monomer of Spx can productively interact with RNA polymerase in Bacillus subtilis.
J Bacteriol: 2012, 194(7);1697-707
[PubMed:22307755]
[WorldCat.org]
[DOI]
(I p)
Shu Ishikawa, Taku Oshima, Ken Kurokawa, Yoko Kusuya, Naotake Ogasawara
RNA polymerase trafficking in Bacillus subtilis cells.
J Bacteriol: 2010, 192(21);5778-87
[PubMed:20817769]
[WorldCat.org]
[DOI]
(I p)
Michiko M Nakano, Ann Lin, Cole S Zuber, Kate J Newberry, Richard G Brennan, Peter Zuber
Promoter recognition by a complex of Spx and the C-terminal domain of the RNA polymerase alpha subunit.
PLoS One: 2010, 5(1);e8664
[PubMed:20084284]
[WorldCat.org]
[DOI]
(I e)
Andreas Licht, Sabine Brantl
The transcriptional repressor CcpN from Bacillus subtilis uses different repression mechanisms at different promoters.
J Biol Chem: 2009, 284(44);30032-8
[PubMed:19726675]
[WorldCat.org]
[DOI]
(I p)
Valerie Lamour, Lars F Westblade, Elizabeth A Campbell, Seth A Darst
Crystal structure of the in vivo-assembled Bacillus subtilis Spx/RNA polymerase alpha subunit C-terminal domain complex.
J Struct Biol: 2009, 168(2);352-6
[PubMed:19580872]
[WorldCat.org]
[DOI]
(I p)
Alexander Reder, Dirk Höper, Christin Weinberg, Ulf Gerth, Martin Fraunholz, Michael Hecker
The Spx paralogue MgsR (YqgZ) controls a subregulon within the general stress response of Bacillus subtilis.
Mol Microbiol: 2008, 69(5);1104-20
[PubMed:18643936]
[WorldCat.org]
[DOI]
(I p)
Ying Zhang, Shunji Nakano, Soon-Yong Choi, Peter Zuber
Mutational analysis of the Bacillus subtilis RNA polymerase alpha C-terminal domain supports the interference model of Spx-dependent repression.
J Bacteriol: 2006, 188(12);4300-11
[PubMed:16740936]
[WorldCat.org]
[DOI]
(P p)
Kate J Newberry, Shunji Nakano, Peter Zuber, Richard G Brennan
Crystal structure of the Bacillus subtilis anti-alpha, global transcriptional regulator, Spx, in complex with the alpha C-terminal domain of RNA polymerase.
Proc Natl Acad Sci U S A: 2005, 102(44);15839-44
[PubMed:16249335]
[WorldCat.org]
[DOI]
(P p)
Christine Eymann, Annette Dreisbach, Dirk Albrecht, Jörg Bernhardt, Dörte Becher, Sandy Gentner, Le Thi Tam, Knut Büttner, Gerrit Buurman, Christian Scharf, Simone Venz, Uwe Völker, Michael Hecker
A comprehensive proteome map of growing Bacillus subtilis cells.
Proteomics: 2004, 4(10);2849-76
[PubMed:15378759]
[WorldCat.org]
[DOI]
(P p)
Shunji Nakano, Michiko M Nakano, Ying Zhang, Montira Leelakriangsak, Peter Zuber
A regulatory protein that interferes with activator-stimulated transcription in bacteria.
Proc Natl Acad Sci U S A: 2003, 100(7);4233-8
[PubMed:12642660]
[WorldCat.org]
[DOI]
(P p)
Claudia Rollenhagen, Haike Antelmann, Janine Kirstein, Olivier Delumeau, Michael Hecker, Michael D Yudkin
Binding of sigma(A) and sigma(B) to core RNA polymerase after environmental stress in Bacillus subtilis.
J Bacteriol: 2003, 185(1);35-40
[PubMed:12486038]
[WorldCat.org]
[DOI]
(P p)
P J Lewis, S D Thaker, J Errington
Compartmentalization of transcription and translation in Bacillus subtilis.
EMBO J: 2000, 19(4);710-8
[PubMed:10675340]
[WorldCat.org]
[DOI]
(P p)
J W Suh, S A Boylan, S H Oh, C W Price
Genetic and transcriptional organization of the Bacillus subtilis spc-alpha region.
Gene: 1996, 169(1);17-23
[PubMed:8635744]
[WorldCat.org]
[DOI]
(P p)
S A Boylan, J W Suh, S M Thomas, C W Price
Gene encoding the alpha core subunit of Bacillus subtilis RNA polymerase is cotranscribed with the genes for initiation factor 1 and ribosomal proteins B, S13, S11, and L17.
J Bacteriol: 1989, 171(5);2553-62
[PubMed:2496109]
[WorldCat.org]
[DOI]
(P p)
J W Suh, S A Boylan, C W Price
Gene for the alpha subunit of Bacillus subtilis RNA polymerase maps in the ribosomal protein gene cluster.
J Bacteriol: 1986, 168(1);65-71
[PubMed:3093467]
[WorldCat.org]
[DOI]
(P p)