RplK
- Description: ribosomal protein
| Gene name | rplK |
| Synonyms | relC, tsp |
| Essential | no PubMed |
| Product | ribosomal protein L11 (BL11) |
| Function | translation |
| Gene expression levels in SubtiExpress: rplK | |
| Interactions involving this protein in SubtInteract: RplK | |
| MW, pI | 14 kDa, 9.718 |
| Gene length, protein length | 423 bp, 141 aa |
| Immediate neighbours | nusG, rplA |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 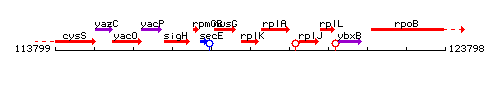
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed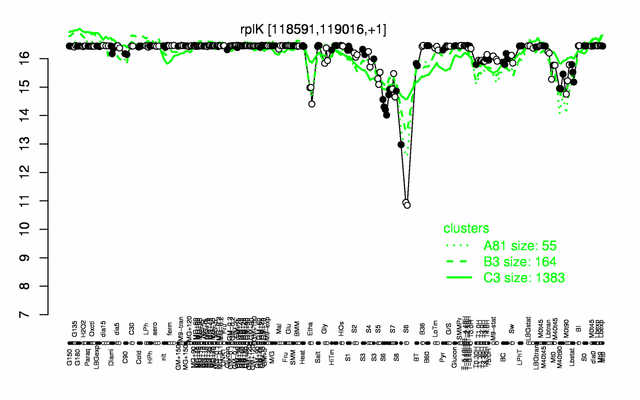
| |
Contents
Categories containing this gene/protein
translation, universally conserved proteins, phosphoproteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU01020
Phenotypes of a mutant
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: ribosomal protein L11P family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- phosphorylated on Arg-94 PubMed
- Cofactor(s):
- Effectors of protein activity:
Database entries
- Structure: 1FOX (C-terminal domain, Geobacillus stearothermophilus), 2FOW (RNA binding domain in complex with RNA, Geobacillus stearothermophilus)
- UniProt: Q06796
- KEGG entry: [2]
- E.C. number:
Additional information
Expression and regulation
- Operon:
- Sigma factor:
- Regulatory mechanism:
- Additional information:
- The mRNA has a long 5' leader region. This may indicate RNA-based regulation PubMed
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Additional publications: PubMed
Alexander K W Elsholz, Kürsad Turgay, Stephan Michalik, Bernd Hessling, Katrin Gronau, Dan Oertel, Ulrike Mäder, Jörg Bernhardt, Dörte Becher, Michael Hecker, Ulf Gerth
Global impact of protein arginine phosphorylation on the physiology of Bacillus subtilis.
Proc Natl Acad Sci U S A: 2012, 109(19);7451-6
[PubMed:22517742]
[WorldCat.org]
[DOI]
(I p)
Irnov Irnov, Cynthia M Sharma, Jörg Vogel, Wade C Winkler
Identification of regulatory RNAs in Bacillus subtilis.
Nucleic Acids Res: 2010, 38(19);6637-51
[PubMed:20525796]
[WorldCat.org]
[DOI]
(I p)
Sascha Baumann, Sebastian Schoof, Marcel Bolten, Claudia Haering, Motoki Takagi, Kazuo Shin-ya, Hans-Dieter Arndt
Molecular determinants of microbial resistance to thiopeptide antibiotics.
J Am Chem Soc: 2010, 132(20);6973-81
[PubMed:20441189]
[WorldCat.org]
[DOI]
(I p)
Matthew A Lauber, William E Running, James P Reilly
B. subtilis ribosomal proteins: structural homology and post-translational modifications.
J Proteome Res: 2009, 8(9);4193-206
[PubMed:19653700]
[WorldCat.org]
[DOI]
(P p)
Christine Eymann, Georg Homuth, Christian Scharf, Michael Hecker
Bacillus subtilis functional genomics: global characterization of the stringent response by proteome and transcriptome analysis.
J Bacteriol: 2002, 184(9);2500-20
[PubMed:11948165]
[WorldCat.org]
[DOI]
(P p)
S Zhang, J M Scott, W G Haldenwang
Loss of ribosomal protein L11 blocks stress activation of the Bacillus subtilis transcription factor sigma(B).
J Bacteriol: 2001, 183(7);2316-21
[PubMed:11244072]
[WorldCat.org]
[DOI]
(P p)
B Wienen, R Ehrlich, M Stöffler-Meilicke, G Stöffler, I Smith, D Weiss, R Vince, S Pestka
Ribosomal protein alterations in thiostrepton- and Micrococcin-resistant mutants of Bacillus subtilis.
J Biol Chem: 1979, 254(16);8031-41
[PubMed:112097]
[WorldCat.org]
(P p)