Difference between revisions of "RecO"
| Line 22: | Line 22: | ||
|style="background:#ABCDEF;" align="center"| '''Gene length, protein length''' || 765 bp, 255 aa | |style="background:#ABCDEF;" align="center"| '''Gene length, protein length''' || 765 bp, 255 aa | ||
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[glyQ]]'', ''[[ | + | |style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[glyQ]]'', ''[[yqzL]]'' |
|- | |- | ||
|colspan="2" style="background:#FAF8CC;" align="center"|'''Get the DNA and protein [http://srs.ebi.ac.uk/srsbin/cgi-bin/wgetz?-e+[EMBLCDS:CAB14457]+-newId sequences] <br/> (Barbe ''et al.'', 2009)''' | |colspan="2" style="background:#FAF8CC;" align="center"|'''Get the DNA and protein [http://srs.ebi.ac.uk/srsbin/cgi-bin/wgetz?-e+[EMBLCDS:CAB14457]+-newId sequences] <br/> (Barbe ''et al.'', 2009)''' | ||
Revision as of 14:53, 3 December 2012
- Description: mediator of RecA binding to ssDNA
| Gene name | recO |
| Synonyms | yqxN, yqfI |
| Essential | no |
| Product | mediator of RecA binding to ssDNA |
| Function | DNA repair/ recombination |
| Gene expression levels in SubtiExpress: recO | |
| Interactions involving this protein in SubtInteract: RecO | |
| MW, pI | 29 kDa, 8.208 |
| Gene length, protein length | 765 bp, 255 aa |
| Immediate neighbours | glyQ, yqzL |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 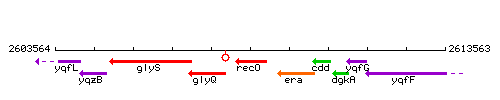
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU25280
Phenotypes of a mutant
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: recO family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization:
- cytoplasm (according to Swiss-Prot)
- localizes to the DNA entry pole during transformation PubMed
Database entries
- Structure:
- UniProt: P42095
- KEGG entry: [2]
- E.C. number:
Additional information
Expression and regulation
- Operon:
- Sigma factor:
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Additional publications: PubMed
Tribhuwan Yadav, Begoña Carrasco, Angela R Myers, Nicholas P George, James L Keck, Juan C Alonso
Genetic recombination in Bacillus subtilis: a division of labor between two single-strand DNA-binding proteins.
Nucleic Acids Res: 2012, 40(12);5546-59
[PubMed:22373918]
[WorldCat.org]
[DOI]
(I p)
Candela Manfredi, Yuki Suzuki, Tribhuwan Yadav, Kunio Takeyasu, Juan C Alonso
RecO-mediated DNA homology search and annealing is facilitated by SsbA.
Nucleic Acids Res: 2010, 38(20);6920-9
[PubMed:20581116]
[WorldCat.org]
[DOI]
(I p)
Dawit Kidane, Begoña Carrasco, Candela Manfredi, Katharina Rothmaier, Silvia Ayora, Serkalem Tadesse, Juan C Alonso, Peter L Graumann
Evidence for different pathways during horizontal gene transfer in competent Bacillus subtilis cells.
PLoS Genet: 2009, 5(9);e1000630
[PubMed:19730681]
[WorldCat.org]
[DOI]
(I p)
Candela Manfredi, Begoña Carrasco, Silvia Ayora, Juan C Alonso
Bacillus subtilis RecO nucleates RecA onto SsbA-coated single-stranded DNA.
J Biol Chem: 2008, 283(36);24837-47
[PubMed:18599486]
[WorldCat.org]
[DOI]
(P p)
Joanna Timmins, Ingar Leiros, Sean McSweeney
Crystal structure and mutational study of RecOR provide insight into its mode of DNA binding.
EMBO J: 2007, 26(13);3260-71
[PubMed:17581636]
[WorldCat.org]
[DOI]
(P p)