Difference between revisions of "RbgA"
| Line 54: | Line 54: | ||
* essential [http://www.ncbi.nlm.nih.gov/pubmed/12682299 PubMed] | * essential [http://www.ncbi.nlm.nih.gov/pubmed/12682299 PubMed] | ||
* depletion confers a slow growth phenotype, and induces an abnormality of the ''in vivo'' ribosome profile, with an increased level of 45S precursors and a decreased level of 70S ribosomes | * depletion confers a slow growth phenotype, and induces an abnormality of the ''in vivo'' ribosome profile, with an increased level of 45S precursors and a decreased level of 70S ribosomes | ||
| − | * premature 45S particles accumulated in YlqF-depleted cells lack several proteins, including [[rplP|L16]], [[ | + | * premature 45S particles accumulated in YlqF-depleted cells lack several proteins, including [[rplP|L16]], [[rpmB|L28]], [[rpmGA|L33]], [[rpmI|L35]] and [[rpmJ|L36]] {{PubMed|23700310}} |
| + | |||
=== Database entries === | === Database entries === | ||
Revision as of 10:23, 12 September 2013
- Description: assembly of the 50S subunit of the ribosome
| Gene name | rbgA |
| Synonyms | ylqF |
| Essential | yes PubMed |
| Product | GTPase |
| Function | ribosome assembly |
| Gene expression levels in SubtiExpress: rbgA | |
| MW, pI | 31 kDa, 9.528 |
| Gene length, protein length | 846 bp, 282 aa |
| Immediate neighbours | rplS, rnhB |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed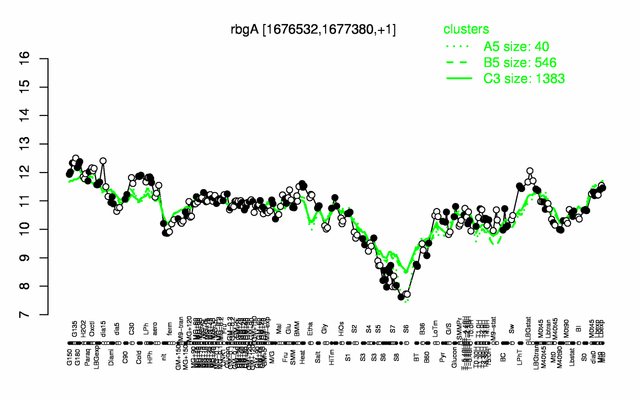
| |
Contents
Categories containing this gene/protein
translation, essential genes, GTP-binding proteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU16050
Phenotypes of a mutant
- essential PubMed
- depletion confers a slow growth phenotype, and induces an abnormality of the in vivo ribosome profile, with an increased level of 45S precursors and a decreased level of 70S ribosomes
- premature 45S particles accumulated in YlqF-depleted cells lack several proteins, including L16, L28, L33, L35 and L36 PubMed
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: Binds and hydrolyzes GTP and readily exchanges GDP for GTP
- Protein family: MTG1 subfamily (according to Swiss-Prot) Era/Obg family
Extended information on the protein
- Kinetic information:
- Domains:
- RNA-binding domain at the C-terminus PubMed
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization: cytoplasm (according to Swiss-Prot)
Database entries
- Structure: 1PUJ
- UniProt: O31743
- KEGG entry: [2]
- E.C. number:
Additional information
Expression and regulation
- Operon:
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Naotake Ogasawara, Nara, Japan
Your additional remarks
References
Reviews
Natalie Verstraeten, Maarten Fauvart, Wim Versées, Jan Michiels
The universally conserved prokaryotic GTPases.
Microbiol Mol Biol Rev: 2011, 75(3);507-42, second and third pages of table of contents
[PubMed:21885683]
[WorldCat.org]
[DOI]
(I p)
Robert A Britton
Role of GTPases in bacterial ribosome assembly.
Annu Rev Microbiol: 2009, 63;155-76
[PubMed:19575570]
[WorldCat.org]
[DOI]
(I p)
Original publications
Ningning Li, Yuling Chen, Qiang Guo, Yixiao Zhang, Yi Yuan, Chengying Ma, Haiteng Deng, Jianlin Lei, Ning Gao
Cryo-EM structures of the late-stage assembly intermediates of the bacterial 50S ribosomal subunit.
Nucleic Acids Res: 2013, 41(14);7073-83
[PubMed:23700310]
[WorldCat.org]
[DOI]
(I p)
Megha Gulati, Nikhil Jain, Baskaran Anand, Balaji Prakash, Robert A Britton
Mutational analysis of the ribosome assembly GTPase RbgA provides insight into ribosome interaction and ribosome-stimulated GTPase activation.
Nucleic Acids Res: 2013, 41(5);3217-27
[PubMed:23325847]
[WorldCat.org]
[DOI]
(I p)
David Achila, Megha Gulati, Nikhil Jain, Robert A Britton
Biochemical characterization of ribosome assembly GTPase RbgA in Bacillus subtilis.
J Biol Chem: 2012, 287(11);8417-23
[PubMed:22267738]
[WorldCat.org]
[DOI]
(I p)
Laura Schaefer, William C Uicker, Catherine Wicker-Planquart, Anne-Emmanuelle Foucher, Jean-Michel Jault, Robert A Britton
Multiple GTPases participate in the assembly of the large ribosomal subunit in Bacillus subtilis.
J Bacteriol: 2006, 188(23);8252-8
[PubMed:16997968]
[WorldCat.org]
[DOI]
(P p)
William C Uicker, Laura Schaefer, Robert A Britton
The essential GTPase RbgA (YlqF) is required for 50S ribosome assembly in Bacillus subtilis.
Mol Microbiol: 2006, 59(2);528-40
[PubMed:16390447]
[WorldCat.org]
[DOI]
(P p)
Takuya Morimoto, Pek Chin Loh, Tomohiro Hirai, Kei Asai, Kazuo Kobayashi, Shigeki Moriya, Naotake Ogasawara
Six GTP-binding proteins of the Era/Obg family are essential for cell growth in Bacillus subtilis.
Microbiology (Reading): 2002, 148(Pt 11);3539-3552
[PubMed:12427945]
[WorldCat.org]
[DOI]
(P p)