Difference between revisions of "RacE"
| Line 123: | Line 123: | ||
* '''Additional information:''' | * '''Additional information:''' | ||
| + | ** number of protein molecules per cell (complex medium with amino acids, without glucose): 531 {{PubMed|24696501}} | ||
=Biological materials = | =Biological materials = | ||
Revision as of 10:09, 17 April 2014
- Description: glutamate racemase
| Gene name | racE |
| Synonyms | glr |
| Essential | yes PubMed |
| Product | glutamate racemase |
| Function | peptidoglycan precursor biosynthesis |
| Gene expression levels in SubtiExpress: racE | |
| Metabolic function and regulation of this protein in SubtiPathways: racE | |
| MW, pI | 29 kDa, 4.858 |
| Gene length, protein length | 816 bp, 272 aa |
| Immediate neighbours | gerM, ysmB |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 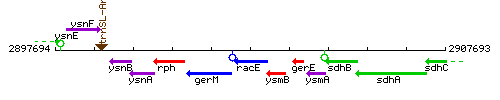
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
cell wall synthesis, biosynthesis of cell wall components, essential genes
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU28390
Phenotypes of a mutant
essential PubMed
Database entries
- BsubCyc: BSU28390
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: L-glutamate = D-glutamate (according to Swiss-Prot)
- Protein family: aspartate/glutamate racemases family (according to Swiss-Prot)
- Paralogous protein(s): YrpC
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- BsubCyc: BSU28390
- Structure: 1ZUW (with D-glutamate)
- UniProt: P94556
- KEGG entry: [2]
- E.C. number: 5.1.1.3
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
- Additional information:
- number of protein molecules per cell (complex medium with amino acids, without glucose): 531 PubMed
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Katie L Whalen, Anthony C Chau, M Ashley Spies
In silico optimization of a fragment-based hit yields biologically active, high-efficiency inhibitors for glutamate racemase.
ChemMedChem: 2013, 8(10);1681-9
[PubMed:23929705]
[WorldCat.org]
[DOI]
(I p)
Pierre Nicolas, Ulrike Mäder, Etienne Dervyn, Tatiana Rochat, Aurélie Leduc, Nathalie Pigeonneau, Elena Bidnenko, Elodie Marchadier, Mark Hoebeke, Stéphane Aymerich, Dörte Becher, Paola Bisicchia, Eric Botella, Olivier Delumeau, Geoff Doherty, Emma L Denham, Mark J Fogg, Vincent Fromion, Anne Goelzer, Annette Hansen, Elisabeth Härtig, Colin R Harwood, Georg Homuth, Hanne Jarmer, Matthieu Jules, Edda Klipp, Ludovic Le Chat, François Lecointe, Peter Lewis, Wolfram Liebermeister, Anika March, Ruben A T Mars, Priyanka Nannapaneni, David Noone, Susanne Pohl, Bernd Rinn, Frank Rügheimer, Praveen K Sappa, Franck Samson, Marc Schaffer, Benno Schwikowski, Leif Steil, Jörg Stülke, Thomas Wiegert, Kevin M Devine, Anthony J Wilkinson, Jan Maarten van Dijl, Michael Hecker, Uwe Völker, Philippe Bessières, Philippe Noirot
Condition-dependent transcriptome reveals high-level regulatory architecture in Bacillus subtilis.
Science: 2012, 335(6072);1103-6
[PubMed:22383849]
[WorldCat.org]
[DOI]
(I p)
M Ashley Spies, Joseph G Reese, Dylan Dodd, Katherine L Pankow, Steven R Blanke, Jerome Baudry
Determinants of catalytic power and ligand binding in glutamate racemase.
J Am Chem Soc: 2009, 131(14);5274-84
[PubMed:19309142]
[WorldCat.org]
[DOI]
(I p)
Eduard Puig, Edgar Mixcoha, Mireia Garcia-Viloca, Angels González-Lafont, José M Lluch
How the substrate D-glutamate drives the catalytic action of Bacillus subtilis glutamate racemase.
J Am Chem Soc: 2009, 131(10);3509-21
[PubMed:19227983]
[WorldCat.org]
[DOI]
(I p)
Sergey N Ruzheinikov, Makie A Taal, Svetlana E Sedelnikova, Patrick J Baker, David W Rice
Substrate-induced conformational changes in Bacillus subtilis glutamate racemase and their implications for drug discovery.
Structure: 2005, 13(11);1707-13
[PubMed:16271894]
[WorldCat.org]
[DOI]
(P p)
Keitarou Kimura, Lam-Son Phan Tran, Yoshifumi Itoh
Roles and regulation of the glutamate racemase isogenes, racE and yrpC, in Bacillus subtilis.
Microbiology (Reading): 2004, 150(Pt 9);2911-2920
[PubMed:15347750]
[WorldCat.org]
[DOI]
(P p)