PyrR
- Description: transcriptional antiterminator of the pyr operon
| Gene name | pyrR |
| Synonyms | |
| Essential | no |
| Product | transcriptional antiterminator with minor uracil phosphoribosyltransferase activity |
| Function | regulation of pyrimidine biosynthesis |
| MW, pI | 20 kDa, 4.991 |
| Gene length, protein length | 543 bp, 181 aa |
| Immediate neighbours | ylyB, pyrP |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 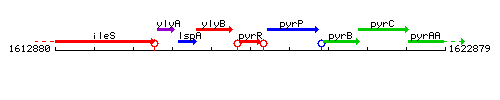
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU15470
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: UMP + diphosphate = uracil + 5-phospho-alpha-D-ribose 1-diphosphate (according to Swiss-Prot)
- Protein family: PyrR subfamily (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization:
Database entries
- Structure: 1A3C (dimeric form)
- Swiss prot entry: P39765
- KEGG entry: [3]
- E.C. number: 2.4.2.9
Additional information
Expression and regulation
- Regulatory mechanism:
- PyrR: RNA switch, transcription termination/ antitermination (in the presence of uridine nucleotides: termination, in their absence: antitermination) PubMed
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Preethi Chander, Kari M Halbig, Jamie K Miller, Christopher J Fields, Heather K S Bonner, Gail K Grabner, Robert L Switzer, Janet L Smith
Structure of the nucleotide complex of PyrR, the pyr attenuation protein from Bacillus caldolyticus, suggests dual regulation by pyrimidine and purine nucleotides.
J Bacteriol: 2005, 187(5);1773-82
[PubMed:15716449]
[WorldCat.org]
[DOI]
(P p)
Hesheng Zhang, Robert L Switzer
Transcriptional pausing in the Bacillus subtilis pyr operon in vitro: a role in transcriptional attenuation?
J Bacteriol: 2003, 185(16);4764-71
[PubMed:12896995]
[WorldCat.org]
[DOI]
(P p)
Gail K Grabner, Robert L Switzer
Kinetic studies of the uracil phosphoribosyltransferase reaction catalyzed by the Bacillus subtilis pyrimidine attenuation regulatory protein PyrR.
J Biol Chem: 2003, 278(9);6921-7
[PubMed:12482852]
[WorldCat.org]
[DOI]
(P p)
Heather K Savacool, Robert L Switzer
Characterization of the interaction of Bacillus subtilis PyrR with pyr mRNA by site-directed mutagenesis of the protein.
J Bacteriol: 2002, 184(9);2521-8
[PubMed:11948166]
[WorldCat.org]
[DOI]
(P p)
E R Bonner, J N D'Elia, B K Billips, R L Switzer
Molecular recognition of pyr mRNA by the Bacillus subtilis attenuation regulatory protein PyrR.
Nucleic Acids Res: 2001, 29(23);4851-65
[PubMed:11726695]
[WorldCat.org]
[DOI]
(I p)
R L Switzer, R J Turner, Y Lu
Regulation of the Bacillus subtilis pyrimidine biosynthetic operon by transcriptional attenuation: control of gene expression by an mRNA-binding protein.
Prog Nucleic Acid Res Mol Biol: 1999, 62;329-67
[PubMed:9932459]
[WorldCat.org]
[DOI]
(P p)
D R Tomchick, R J Turner, R L Switzer, J L Smith
Adaptation of an enzyme to regulatory function: structure of Bacillus subtilis PyrR, a pyr RNA-binding attenuation protein and uracil phosphoribosyltransferase.
Structure: 1998, 6(3);337-50
[PubMed:9551555]
[WorldCat.org]
[DOI]
(P p)
R J Turner, E R Bonner, G K Grabner, R L Switzer
Purification and characterization of Bacillus subtilis PyrR, a bifunctional pyr mRNA-binding attenuation protein/uracil phosphoribosyltransferase.
J Biol Chem: 1998, 273(10);5932-8
[PubMed:9488732]
[WorldCat.org]
[DOI]
(P p)
Y Lu, R L Switzer
Transcriptional attenuation of the Bacillus subtilis pyr operon by the PyrR regulatory protein and uridine nucleotides in vitro.
J Bacteriol: 1996, 178(24);7206-11
[PubMed:8955403]
[WorldCat.org]
[DOI]
(P p)
Y Lu, R L Switzer
Evidence that the Bacillus subtilis pyrimidine regulatory protein PyrR acts by binding to pyr mRNA at three sites in vivo.
J Bacteriol: 1996, 178(19);5806-9
[PubMed:8824632]
[WorldCat.org]
[DOI]
(P p)
A E Kahler, R L Switzer
Identification of a novel gene of pyrimidine nucleotide biosynthesis, pyrDII, that is required for dihydroorotate dehydrogenase activity in Bacillus subtilis.
J Bacteriol: 1996, 178(16);5013-6
[PubMed:8759868]
[WorldCat.org]
[DOI]
(P p)
S Y Ghim, R L Switzer
Mutations in Bacillus subtilis PyrR, the pyr regulatory protein, with defects in regulation by pyrimidines.
FEMS Microbiol Lett: 1996, 137(1);13-8
[PubMed:8935652]
[WorldCat.org]
[DOI]
(P p)
Y Lu, R J Turner, R L Switzer
Roles of the three transcriptional attenuators of the Bacillus subtilis pyrimidine biosynthetic operon in the regulation of its expression.
J Bacteriol: 1995, 177(5);1315-25
[PubMed:7868607]
[WorldCat.org]
[DOI]
(P p)
J Martinussen, P Glaser, P S Andersen, H H Saxild
Two genes encoding uracil phosphoribosyltransferase are present in Bacillus subtilis.
J Bacteriol: 1995, 177(1);271-4
[PubMed:7798145]
[WorldCat.org]
[DOI]
(P p)
R J Turner, Y Lu, R L Switzer
Regulation of the Bacillus subtilis pyrimidine biosynthetic (pyr) gene cluster by an autogenous transcriptional attenuation mechanism.
J Bacteriol: 1994, 176(12);3708-22
[PubMed:8206849]
[WorldCat.org]
[DOI]
(P p)
C L Quinn, B T Stephenson, R L Switzer
Functional organization and nucleotide sequence of the Bacillus subtilis pyrimidine biosynthetic operon.
J Biol Chem: 1991, 266(14);9113-27
[PubMed:1709162]
[WorldCat.org]
(P p)