Difference between revisions of "PurS"
| Line 25: | Line 25: | ||
|style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[purC]]'', ''[[purQ]]'' | |style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[purC]]'', ''[[purQ]]'' | ||
|- | |- | ||
| − | |style="background:#FAF8CC;" align="center"|'''Sequences'''||[http://bsubcyc.org/BSUB/sequence-aa?type=GENE&object=BSU06460 Protein] [http://bsubcyc.org/BSUB/sequence?type=GENE&object=BSU06460 DNA] [http://bsubcyc.org/BSUB/seq-selector?chromosome=CHROM-1&object=BSU06460 | + | |style="background:#FAF8CC;" align="center"|'''Sequences'''||[http://bsubcyc.org/BSUB/sequence-aa?type=GENE&object=BSU06460 Protein] [http://bsubcyc.org/BSUB/sequence?type=GENE&object=BSU06460 DNA] [http://bsubcyc.org/BSUB/seq-selector?chromosome=CHROM-1&object=BSU06460 DNA_with_flanks] |
|- | |- | ||
|colspan="2" | '''Genetic context''' <br/> [[Image:purS_context.gif]] | |colspan="2" | '''Genetic context''' <br/> [[Image:purS_context.gif]] | ||
Revision as of 10:37, 14 May 2013
- Description: phosphoribosylformylglycinamidine synthase
| Gene name | purS |
| Synonyms | yexA |
| Essential | no |
| Product | phosphoribosylformylglycinamidine synthase |
| Function | purine biosynthesis |
| Gene expression levels in SubtiExpress: purS | |
| Interactions involving this protein in SubtInteract: PurS | |
| Metabolic function and regulation of this protein in SubtiPathways: Purine synthesis, Nucleotides (regulation), Stress | |
| MW, pI | 9 kDa, 4.594 |
| Gene length, protein length | 252 bp, 84 aa |
| Immediate neighbours | purC, purQ |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed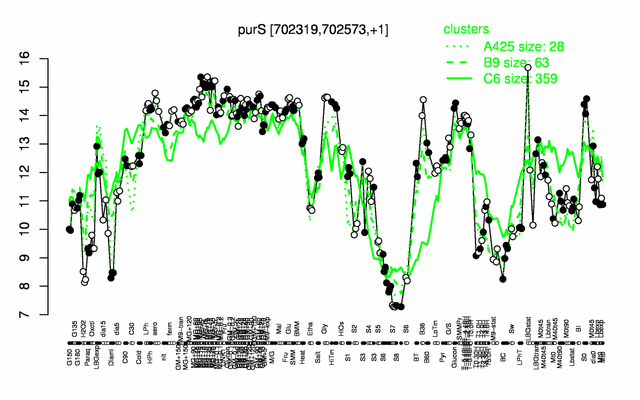
| |
Contents
Categories containing this gene/protein
biosynthesis/ acquisition of nucleotides
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU06460
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: UPF0062 family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- Structure: 1T4A
- UniProt: P12049
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Ruchi Anand, Aaron A Hoskins, Eric M Bennett, Michael D Sintchak, JoAnne Stubbe, Steven E Ealick
A model for the Bacillus subtilis formylglycinamide ribonucleotide amidotransferase multiprotein complex.
Biochemistry: 2004, 43(32);10343-52
[PubMed:15301532]
[WorldCat.org]
[DOI]
(P p)
Aaron A Hoskins, Ruchi Anand, Steven E Ealick, JoAnne Stubbe
The formylglycinamide ribonucleotide amidotransferase complex from Bacillus subtilis: metabolite-mediated complex formation.
Biochemistry: 2004, 43(32);10314-27
[PubMed:15301530]
[WorldCat.org]
[DOI]
(P p)
Hans H Saxild, Per Nygaard
The yexA gene product is required for phosphoribosylformylglycinamidine synthetase activity in Bacillus subtilis.
Microbiology (Reading): 2000, 146 ( Pt 4);807-814
[PubMed:10784038]
[WorldCat.org]
[DOI]
(P p)
M Weng, P L Nagy, H Zalkin
Identification of the Bacillus subtilis pur operon repressor.
Proc Natl Acad Sci U S A: 1995, 92(16);7455-9
[PubMed:7638212]
[WorldCat.org]
[DOI]
(P p)
D J Ebbole, H Zalkin
Cloning and characterization of a 12-gene cluster from Bacillus subtilis encoding nine enzymes for de novo purine nucleotide synthesis.
J Biol Chem: 1987, 262(17);8274-87
[PubMed:3036807]
[WorldCat.org]
(P p)