Difference between revisions of "PtsI"
(→Database entries) |
|||
| Line 98: | Line 98: | ||
**''[[ptsH]]-[[ptsI]]'' | **''[[ptsH]]-[[ptsI]]'' | ||
| − | * '''Sigma factor:''' [[SigA]] [http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=+11902727 PubMed] | + | * '''[[Sigma factor]]:''' [[SigA]] [http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=+11902727 PubMed] |
* '''Regulation:''' expression activated by glucose (4.3 fold) [http://www.ncbi.nlm.nih.gov/pubmed/12850135 PubMed], induction by glucose (''[[ptsG]]''), constitutive (''[[ptsH]]'') | * '''Regulation:''' expression activated by glucose (4.3 fold) [http://www.ncbi.nlm.nih.gov/pubmed/12850135 PubMed], induction by glucose (''[[ptsG]]''), constitutive (''[[ptsH]]'') | ||
Revision as of 10:55, 8 April 2009
- Description: Enzyme I, general (non sugar-specific) component of the PTS. Enzyme I transfers the phosphoryl group from phosphoenolpyruvate (PEP) to the phosphoryl carrier protein (HPr)
| Gene name | ptsI |
| Synonyms | |
| Essential | no |
| Product | phosphotransferase system (PTS) enzyme I |
| Function | PTS-dependent sugar transport |
| MW, pI | 62,9 kDa, 4.59 |
| Gene length, protein length | 1710 bp, 570 amino acids |
| Immediate neighbours | ptsH, splA |
| Gene sequence (+200bp) | Protein sequence |
| Caution: The sequence for this gene in SubtiList contains errors | |
Genetic context 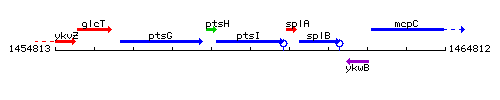
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Coordinates: 1458959 - 1460668
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: PEP-dependent autophosphorylation on His-189, transfer of the phosphoryl group to HPr (His-15)
- Protein family: PEP-utilizing enzyme family
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- HPr binding site (N-Terminal Domain)
- pyruvate binding site (C-Terminal Domain)
- pyrophosphate/phosphate carrier histidine (central Domain)
- Modification: transient autophosphorylation on His-189, in vivo also phosphorylated on Ser-34 or Ser-36 PubMed
- Cofactor(s): Magnesium
- Effectors of protein activity:
- Interactions:
- Localization: Cytoplasm
Database entries
- Structure:
- Swiss prot entry: [3]
- KEGG entry: [4]
- E.C. number: 2.7.3.9
Additional information
Expression and regulation
- Sigma factor: SigA PubMed
- Regulation: expression activated by glucose (4.3 fold) PubMed, induction by glucose (ptsG), constitutive (ptsH)
- Additional information:
Biological materials
- Mutant: GP864 (ermC), available in Stülke lab
- Expression vector: pAG3 (His-tag), available in Galinier lab
- lacZ fusion:
- GFP fusion:
- Antibody:
Labs working on this gene/protein
Josef Deutscher, Paris-Grignon, France
Jörg Stülke, University of Göttingen, Germany Homepage
Your additional remarks
References
- Blencke et al. (2003) Transcriptional profiling of gene expression in response to glucose in Bacillus subtilis: regulation of the central metabolic pathways. Metab Eng. 5: 133-149 PubMed
- Frisby, D., and Zuber, P. 1994. Mutations in pts cause catabolite-resistant sporulation and altered regulation of spo0H in Bacillus subtilis. J. Bacteriol. 176: 2587-2595. PubMed
- Macek B, Mijakovic I, Olsen JV (2007) The serine/threonine/tyrosine phosphoproteome of the model bacterium Bacillus subtilis. Mol Cell Proteomics 6(4): 697-707. PubMed