Difference between revisions of "PtsH"
Raphael2215 (talk | contribs) |
|||
| Line 45: | Line 45: | ||
{{SubtiWiki category|[[phosphotransferase systems]]}}, | {{SubtiWiki category|[[phosphotransferase systems]]}}, | ||
{{SubtiWiki category|[[transcription factors and their control]]}}, | {{SubtiWiki category|[[transcription factors and their control]]}}, | ||
| − | {{SubtiWiki category|[[phosphoproteins]]}} | + | {{SubtiWiki category|[[phosphoproteins]]}}, |
| + | [[most abundant proteins]] | ||
= This gene is a member of the following [[regulons]] = | = This gene is a member of the following [[regulons]] = | ||
| Line 66: | Line 67: | ||
=== Additional information=== | === Additional information=== | ||
| − | |||
| − | |||
=The protein= | =The protein= | ||
| Line 83: | Line 82: | ||
* '''Kinetic information:''' | * '''Kinetic information:''' | ||
| − | * '''Domains:''' HPr Domain (2–88) | + | * '''[[Domains]]:''' HPr Domain (2–88) |
* '''Modification:''' | * '''Modification:''' | ||
| Line 92: | Line 91: | ||
** ''in vitro'' phosphorylated by [[PrkC]] on Ser-12 {{PubMed|20389117}} | ** ''in vitro'' phosphorylated by [[PrkC]] on Ser-12 {{PubMed|20389117}} | ||
| − | * ''' | + | * '''[[Cofactors]]:''' |
* '''Effectors of protein activity:''' | * '''Effectors of protein activity:''' | ||
| Line 151: | Line 150: | ||
* '''Additional information:''' | * '''Additional information:''' | ||
| + | ** belongs to the 100 [[most abundant proteins]] {{PubMed|15378759}} | ||
=Biological materials = | =Biological materials = | ||
| Line 195: | Line 195: | ||
=References= | =References= | ||
| − | <pubmed>16519689,,12850135 17218307 16519689 17142398 12359875 1577686 9162046 11929549 9336834 7803390, 7623661, 2846556, 8169206, 9973552, 15126459, 10048041, 12169607, 9622354, 10217795, 17693724, 18757537 , 9202047 , 7592487, 15369672 , 14527945, 2507315 8580838 19651770 8418852 1303754 1549615 20081037 20389117 20444094 22001508 22722928 23551403</pubmed> | + | <pubmed>16519689,,12850135 17218307 16519689 17142398 12359875 1577686 9162046 11929549 9336834 7803390, 7623661, 2846556, 8169206, 9973552, 15126459, 10048041, 12169607, 9622354, 10217795, 17693724, 18757537 , 9202047 , 7592487, 15369672 , 14527945, 2507315 8580838 19651770 8418852 1303754 1549615 20081037 20389117 20444094 22001508 22722928 23551403 15378759</pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 16:16, 5 March 2014
- Description: HPr, General component of the sugar phosphotransferase system (PTS).
| Gene name | ptsH |
| Synonyms | |
| Essential | no |
| Product | histidine-containing phosphocarrier protein HPr of the PTS |
| Function | PTS-dependent sugar transport and carbon catabolite repression |
| Gene expression levels in SubtiExpress: ptsH | |
| Interactions involving this protein in SubtInteract: PtsH | |
| Metabolic function and regulation of this protein in SubtiPathways: PtsH | |
| MW, pI | 9,1 kDa, 4.58 |
| Gene length, protein length | 264 bp, 88 amino acids |
| Immediate neighbours | ptsG, ptsI |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 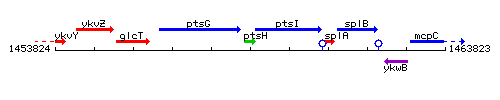
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
phosphotransferase systems, transcription factors and their control, phosphoproteins, most abundant proteins
This gene is a member of the following regulons
GlcT regulon, stringent response
The gene
Basic information
- Locus tag: BSU13900
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry:[2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: Protein HPr N(pi)-phospho-L-histidine + protein EIIA = protein HPr + protein EIIA N(tau)-phospho-L-histidine (according to Swiss-Prot) Protein HPr N(pi)-phospho-L-histidine + protein EIIA = protein HPr + protein EIIA N(tau)-phospho-L-histidine
- Protein family: HPr domain (according to Swiss-Prot) HPr family
- Paralogous protein(s): Crh
Extended information on the protein
- Kinetic information:
- Domains: HPr Domain (2–88)
- Modification:
- Effectors of protein activity:
- Interactions:
- HprK-HPr PubMed
- Enzyme I-HPr
- HPr-PtsG PubMed, PtsH-MtlF PubMed, ManP-HPr, GmuA-HPr
- GamP-HPr, BglP-HPr, LicA-HPr, LevD-HPr
- FruA-HPr, YpqE-HPr
- HPr-LicT, HPr-SacY, HPr-SacT, HPr-GlcT
- HPr-MtlR PubMed, HPr-LicR, HPr-LevR,HPr-ManR
- HPr-GlpK
- GapA-HPr PubMed
- HPr(Ser-46)-P-CcpA PubMed
- HPr-RbsR PubMed
- HPr(His)-P-YesS PubMed
- Localization: cytoplasm PubMed
Database entries
- Structure:
- 1KKM (complex of L. casei HprK with B. subtilis HPr-Ser-P)
- 1KKL (complex of Lactobacillus casei HprK with B. subtilis HPr)
- 2HID (NMR)
- 3OQM (complex of B. subtilis CcpA with P-Ser-HPr and the ackA operator site)
- 3OQN (complex of B. subtilis CcpA with P-Ser-HPr and the gntR operator site)
- 3OQO (complex of B. subtilis CcpA with P-Ser-HPr and a optimal synthetic operator site)
- UniProt: P08877
- KEGG entry: [3]
- E.C. number: 2.7.11.-
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
- ptsG: transcriptional antitermination via the GlcT-dependent RNA switch PubMed
- Additional information:
- belongs to the 100 most abundant proteins PubMed
Biological materials
- Mutant: available in Jörg Stülke's lab:
- Expression vector:
- pGP438 (with N-terminal Strep-tag, in pGP172), available in Jörg Stülke's lab
- pAG2 (His-tag) PubMed, available in Anne Galinier lab
- pGP371(expression / purification of HPr-S46A, with His-tag from E. coli, in pWH844), available in Jörg Stülke's lab
- pGP1415 (HPr, expression in B. subtilis, from pBQ200), available in Jörg Stülke's lab
- pGP961 (HPr, expression in B. subtilis with N-terminal Strep-tag, for SPINE, available in Jörg Stülke's lab
- pGP1416 (HPr-H15A, expression in B. subtilis, from pBQ200), available in Jörg Stülke's lab
- lacZ fusion:
- GFP fusion:
- CFP fusion: B. subtilis GP1267 ptsH-cfp ermC- without terminator, available in Jörg Stülke's lab
- two-hybrid system: B. pertussis adenylate cyclase-based bacterial two hybrid system (BACTH), available in Jörg Stülke's lab
- Antibody: available in Jörg Stülke's lab
Labs working on this gene/protein
Josef Deutscher, Paris-Grignon, France
Jörg Stülke, University of Göttingen, Germany Homepage
Wolfgang Hillen, Erlangen University, Germany Homepage
Richard Brennan, Houston, Texas, USA Homepage
Boris Görke, University of Göttingen, Germany Homepage
Anne Galinier, University of Marseille, France
Your additional remarks
References
Marian Wenzel, Josef Altenbuchner
The Bacillus subtilis mannose regulator, ManR, a DNA-binding protein regulated by HPr and its cognate PTS transporter ManP.
Mol Microbiol: 2013, 88(3);562-76
[PubMed:23551403]
[WorldCat.org]
[DOI]
(I p)
Sebastian Himmel, Christopher P Zschiedrich, Stefan Becker, He-Hsuan Hsiao, Sebastian Wolff, Christine Diethmaier, Henning Urlaub, Donghan Lee, Christian Griesinger, Jörg Stülke
Determinants of interaction specificity of the Bacillus subtilis GlcT antitermination protein: functionality and phosphorylation specificity depend on the arrangement of the regulatory domains.
J Biol Chem: 2012, 287(33);27731-42
[PubMed:22722928]
[WorldCat.org]
[DOI]
(I p)
Frederik M Meyer, Matthieu Jules, Felix M P Mehne, Dominique Le Coq, Jens J Landmann, Boris Görke, Stéphane Aymerich, Jörg Stülke
Malate-mediated carbon catabolite repression in Bacillus subtilis involves the HPrK/CcpA pathway.
J Bacteriol: 2011, 193(24);6939-49
[PubMed:22001508]
[WorldCat.org]
[DOI]
(I p)
Philippe Joyet, Meriem Derkaoui, Sandrine Poncet, Josef Deutscher
Control of Bacillus subtilis mtl operon expression by complex phosphorylation-dependent regulation of the transcriptional activator MtlR.
Mol Microbiol: 2010, 76(5);1279-94
[PubMed:20444094]
[WorldCat.org]
[DOI]
(I p)
Nico Pietack, Dörte Becher, Sebastian R Schmidl, Milton H Saier, Michael Hecker, Fabian M Commichau, Jörg Stülke
In vitro phosphorylation of key metabolic enzymes from Bacillus subtilis: PrkC phosphorylates enzymes from different branches of basic metabolism.
J Mol Microbiol Biotechnol: 2010, 18(3);129-40
[PubMed:20389117]
[WorldCat.org]
[DOI]
(I p)
Shigeo Tojo, Kanako Kumamoto, Kazutake Hirooka, Yasutaro Fujita
Heavy involvement of stringent transcription control depending on the adenine or guanine species of the transcription initiation site in glucose and pyruvate metabolism in Bacillus subtilis.
J Bacteriol: 2010, 192(6);1573-85
[PubMed:20081037]
[WorldCat.org]
[DOI]
(I p)
Sandrine Poncet, Maryline Soret, Peggy Mervelet, Josef Deutscher, Philippe Noirot
Transcriptional activator YesS is stimulated by histidine-phosphorylated HPr of the Bacillus subtilis phosphotransferase system.
J Biol Chem: 2009, 284(41);28188-28197
[PubMed:19651770]
[WorldCat.org]
[DOI]
(I p)
Kalpana D Singh, Matthias H Schmalisch, Jörg Stülke, Boris Görke
Carbon catabolite repression in Bacillus subtilis: quantitative analysis of repression exerted by different carbon sources.
J Bacteriol: 2008, 190(21);7275-84
[PubMed:18757537]
[WorldCat.org]
[DOI]
(I p)
Kalpana D Singh, Sven Halbedel, Boris Görke, Jörg Stülke
Control of the phosphorylation state of the HPr protein of the phosphotransferase system in Bacillus subtilis: implication of the protein phosphatase PrpC.
J Mol Microbiol Biotechnol: 2007, 13(1-3);165-71
[PubMed:17693724]
[WorldCat.org]
[DOI]
(P p)
Boris Macek, Ivan Mijakovic, Jesper V Olsen, Florian Gnad, Chanchal Kumar, Peter R Jensen, Matthias Mann
The serine/threonine/tyrosine phosphoproteome of the model bacterium Bacillus subtilis.
Mol Cell Proteomics: 2007, 6(4);697-707
[PubMed:17218307]
[WorldCat.org]
[DOI]
(P p)
Frédérique Pompeo, Jennifer Luciano, Anne Galinier
Interaction of GapA with HPr and its homologue, Crh: Novel levels of regulation of a key step of glycolysis in Bacillus subtilis?
J Bacteriol: 2007, 189(3);1154-7
[PubMed:17142398]
[WorldCat.org]
[DOI]
(P p)
Wolfgang Müller, Nicola Horstmann, Wolfgang Hillen, Heinrich Sticht
The transcription regulator RbsR represents a novel interaction partner of the phosphoprotein HPr-Ser46-P in Bacillus subtilis.
FEBS J: 2006, 273(6);1251-61
[PubMed:16519689]
[WorldCat.org]
[DOI]
(P p)
Christine Eymann, Annette Dreisbach, Dirk Albrecht, Jörg Bernhardt, Dörte Becher, Sandy Gentner, Le Thi Tam, Knut Büttner, Gerrit Buurman, Christian Scharf, Simone Venz, Uwe Völker, Michael Hecker
A comprehensive proteome map of growing Bacillus subtilis cells.
Proteomics: 2004, 4(10);2849-76
[PubMed:15378759]
[WorldCat.org]
[DOI]
(P p)
Maria A Schumacher, Gregory S Allen, Marco Diel, Gerald Seidel, Wolfgang Hillen, Richard G Brennan
Structural basis for allosteric control of the transcription regulator CcpA by the phosphoprotein HPr-Ser46-P.
Cell: 2004, 118(6);731-41
[PubMed:15369672]
[WorldCat.org]
[DOI]
(P p)
Boris Görke, Laetitia Fraysse, Anne Galinier
Drastic differences in Crh and HPr synthesis levels reflect their different impacts on catabolite repression in Bacillus subtilis.
J Bacteriol: 2004, 186(10);2992-5
[PubMed:15126459]
[WorldCat.org]
[DOI]
(P p)
Matthias H Schmalisch, Steffi Bachem, Jörg Stülke
Control of the Bacillus subtilis antiterminator protein GlcT by phosphorylation. Elucidation of the phosphorylation chain leading to inactivation of GlcT.
J Biol Chem: 2003, 278(51);51108-15
[PubMed:14527945]
[WorldCat.org]
[DOI]
(P p)
Hans-Matti Blencke, Georg Homuth, Holger Ludwig, Ulrike Mäder, Michael Hecker, Jörg Stülke
Transcriptional profiling of gene expression in response to glucose in Bacillus subtilis: regulation of the central metabolic pathways.
Metab Eng: 2003, 5(2);133-49
[PubMed:12850135]
[WorldCat.org]
[DOI]
(P p)
Sonia Fieulaine, Solange Morera, Sandrine Poncet, Ivan Mijakovic, Anne Galinier, Joël Janin, Josef Deutscher, Sylvie Nessler
X-ray structure of a bifunctional protein kinase in complex with its protein substrate HPr.
Proc Natl Acad Sci U S A: 2002, 99(21);13437-41
[PubMed:12359875]
[WorldCat.org]
[DOI]
(P p)
Cordula Lindner, Michael Hecker, Dominique Le Coq, Josef Deutscher
Bacillus subtilis mutant LicT antiterminators exhibiting enzyme I- and HPr-independent antitermination affect catabolite repression of the bglPH operon.
J Bacteriol: 2002, 184(17);4819-28
[PubMed:12169607]
[WorldCat.org]
[DOI]
(P p)
Emmanuelle Darbon, Pascale Servant, Sandrine Poncet, Josef Deutscher
Antitermination by GlpP, catabolite repression via CcpA and inducer exclusion triggered by P-GlpK dephosphorylation control Bacillus subtilis glpFK expression.
Mol Microbiol: 2002, 43(4);1039-52
[PubMed:11929549]
[WorldCat.org]
[DOI]
(P p)
I Martin-Verstraete, J Deutscher, A Galinier
Phosphorylation of HPr and Crh by HprK, early steps in the catabolite repression signalling pathway for the Bacillus subtilis levanase operon.
J Bacteriol: 1999, 181(9);2966-9
[PubMed:10217795]
[WorldCat.org]
[DOI]
(P p)
C Lindner, A Galinier, M Hecker, J Deutscher
Regulation of the activity of the Bacillus subtilis antiterminator LicT by multiple PEP-dependent, enzyme I- and HPr-catalysed phosphorylation.
Mol Microbiol: 1999, 31(3);995-1006
[PubMed:10048041]
[WorldCat.org]
[DOI]
(P p)
A Galinier, J Deutscher, I Martin-Verstraete
Phosphorylation of either crh or HPr mediates binding of CcpA to the bacillus subtilis xyn cre and catabolite repression of the xyn operon.
J Mol Biol: 1999, 286(2);307-14
[PubMed:9973552]
[WorldCat.org]
[DOI]
(P p)
I Martin-Verstraete, V Charrier, J Stülke, A Galinier, B Erni, G Rapoport, J Deutscher
Antagonistic effects of dual PTS-catalysed phosphorylation on the Bacillus subtilis transcriptional activator LevR.
Mol Microbiol: 1998, 28(2);293-303
[PubMed:9622354]
[WorldCat.org]
[DOI]
(P p)
B E Jones, P Rajagopal, R E Klevit
Phosphorylation on histidine is accompanied by localized structural changes in the phosphocarrier protein, HPr from Bacillus subtilis.
Protein Sci: 1997, 6(10);2107-19
[PubMed:9336834]
[WorldCat.org]
[DOI]
(P p)
P Tortosa, S Aymerich, C Lindner, M H Saier, J Reizer, D Le Coq
Multiple phosphorylation of SacY, a Bacillus subtilis transcriptional antiterminator negatively controlled by the phosphotransferase system.
J Biol Chem: 1997, 272(27);17230-7
[PubMed:9202047]
[WorldCat.org]
[DOI]
(P p)
V Charrier, E Buckley, D Parsonage, A Galinier, E Darbon, M Jaquinod, E Forest, J Deutscher, A Claiborne
Cloning and sequencing of two enterococcal glpK genes and regulation of the encoded glycerol kinases by phosphoenolpyruvate-dependent, phosphotransferase system-catalyzed phosphorylation of a single histidyl residue.
J Biol Chem: 1997, 272(22);14166-74
[PubMed:9162046]
[WorldCat.org]
[DOI]
(P p)
K Pullen, P Rajagopal, B R Branchini, M E Huffine, J Reizer, M H Saier, J M Scholtz, R E Klevit
Phosphorylation of serine-46 in HPr, a key regulatory protein in bacteria, results in stabilization of its solution structure.
Protein Sci: 1995, 4(12);2478-86
[PubMed:8580838]
[WorldCat.org]
[DOI]
(P p)
J Stülke, I Martin-Verstraete, V Charrier, A Klier, J Deutscher, G Rapoport
The HPr protein of the phosphotransferase system links induction and catabolite repression of the Bacillus subtilis levanase operon.
J Bacteriol: 1995, 177(23);6928-36
[PubMed:7592487]
[WorldCat.org]
[DOI]
(P p)
J Deutscher, E Küster, U Bergstedt, V Charrier, W Hillen
Protein kinase-dependent HPr/CcpA interaction links glycolytic activity to carbon catabolite repression in gram-positive bacteria.
Mol Microbiol: 1995, 15(6);1049-53
[PubMed:7623661]
[WorldCat.org]
[DOI]
(P p)
P Rajagopal, E B Waygood, R E Klevit
Structural consequences of histidine phosphorylation: NMR characterization of the phosphohistidine form of histidine-containing protein from Bacillus subtilis and Escherichia coli.
Biochemistry: 1994, 33(51);15271-82
[PubMed:7803390]
[WorldCat.org]
[DOI]
(P p)
D Frisby, P Zuber
Mutations in pts cause catabolite-resistant sporulation and altered regulation of spo0H in Bacillus subtilis.
J Bacteriol: 1994, 176(9);2587-95
[PubMed:8169206]
[WorldCat.org]
[DOI]
(P p)
Y Chen, J Reizer, M H Saier, W J Fairbrother, P E Wright
Mapping of the binding interfaces of the proteins of the bacterial phosphotransferase system, HPr and IIAglc.
Biochemistry: 1993, 32(1);32-7
[PubMed:8418852]
[WorldCat.org]
[DOI]
(P p)
M Wittekind, P Rajagopal, B R Branchini, J Reizer, M H Saier, R E Klevit
Solution structure of the phosphocarrier protein HPr from Bacillus subtilis by two-dimensional NMR spectroscopy.
Protein Sci: 1992, 1(10);1363-76
[PubMed:1303754]
[WorldCat.org]
[DOI]
(P p)
M Arnaud, P Vary, M Zagorec, A Klier, M Debarbouille, P Postma, G Rapoport
Regulation of the sacPA operon of Bacillus subtilis: identification of phosphotransferase system components involved in SacT activity.
J Bacteriol: 1992, 174(10);3161-70
[PubMed:1577686]
[WorldCat.org]
[DOI]
(P p)
O Herzberg, P Reddy, S Sutrina, M H Saier, J Reizer, G Kapadia
Structure of the histidine-containing phosphocarrier protein HPr from Bacillus subtilis at 2.0-A resolution.
Proc Natl Acad Sci U S A: 1992, 89(6);2499-503
[PubMed:1549615]
[WorldCat.org]
[DOI]
(P p)
J Reizer, S L Sutrina, M H Saier, G C Stewart, A Peterkofsky, P Reddy
Mechanistic and physiological consequences of HPr(ser) phosphorylation on the activities of the phosphoenolpyruvate:sugar phosphotransferase system in gram-positive bacteria: studies with site-specific mutants of HPr.
EMBO J: 1989, 8(7);2111-20
[PubMed:2507315]
[WorldCat.org]
[DOI]
(P p)
R Eisermann, J Deutscher, G Gonzy-Treboul, W Hengstenberg
Site-directed mutagenesis with the ptsH gene of Bacillus subtilis. Isolation and characterization of heat-stable proteins altered at the ATP-dependent regulatory phosphorylation site.
J Biol Chem: 1988, 263(32);17050-4
[PubMed:2846556]
[WorldCat.org]
(P p)