PtsG
- Description: Major glucose permease of the PTS, EIICBA(Glc)
| Gene name | ptsG |
| Synonyms | ptsX, crr |
| Essential | no |
| Product | glucose-specific enzyme IICBA component |
| Function | glucose transport and phosphorylation |
| MW, pI | 75,3 kDa, 5.40 |
| Gene length, protein length | 2097 bp, 699 amino acids |
| Immediate neighbours | glcT, ptsH |
| Gene sequence (+200bp) | Protein sequence |
Genetic context 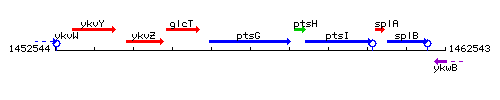
| |
Contents
The gene
Basic information
- Coordinates: 1456496 - 1458592
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: transport and phosphorylation of glucose, receives a phosphate from HPr at the IIA domain (His-620), the phosphate group is then transferred to the IIB domain (Cys-461) an finally to the incoming glucose. In the absence of glucose, PtsG phosphorylates and thereby inactivates the transcriptional antiterminator GlcT.
- Protein family: PTS enzyme II, glucose family
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- 11x transmembrane domain (16–36, 89–109, 139–159, 180–200, 233–253, 283–303, 313–333, 338–358, 365–385, 388–408)
- PTS EIIC domain ( 1-424)
- PTS EIIB domain (439–520)
- PTS EIIA domain (568–672)
- Modification: transient phosphorylation (HPr-dependent) on His-620, then internal phosphotransfer from His-620 to Cys-461
- Cofactor(s):
- Effectors of protein activity:
- Localization: membrane protein NCBI
Database entries
- Swiss prot entry: [3]
- KEGG entry: [4]
- E.C. number: [5]
Additional information
Expression and regulation
- Regulation: induction by glucose
- Regulatory mechanism: transcriptional antitermination via the GlcT-dependent RNA-switch PubMed
- Additional information:
Biological materials
Labs working on this gene/protein
Jörg Stülke, University of Göttingen, Germany Homepage
Your additional remarks
References
- Stülke J, Martin-Verstraete I, Zagorec M (1997) Induction of the Bacillus subtilis ptsGHI operon by glucose is controlled by a novel antiterminator, GlcT Mol Microbiol. 25: 65-78. PubMed
- Bachem S, Stülke J. (1998) Regulation of the Bacillus subtilis GlcT antiterminator protein by components of the phosphotransferase system. J Bacteriol. 180: 5319-26 PubMed
- Bachem, S., Faires, N., & Stülke, J. (1997) Characterization of the presumptive phosphorylation sites of the Bacillus subtilis glucose permease by site-directed mutagenesis: Implication in glucose transport and catabolite repression. FEMS Microbiol. L. 156: 233-238. PubMed
- Gonzy-Tréboul, G., de Waard, J. H., Zagorec, M., and Postma, P.W. (1991). The glucose permease of the phosphotransferase system of Bacillus subtilis: Evidence for IIGlc and IIIGlc domains. Mol. Microbiol. 5, 1241-1249. PubMed
- Langbein, I., Bachem, S. & Stülke, J. (1999) Specific interaction of the RNA binding domain of the Bacillus subtilis transcriptional antiterminator GlcT with its RNA target, RAT. J. Mol. Biol. 293: 795-805. PubMed
- Schilling, O., Herzberg, C., Hertrich, T., Vörsmann, H., Jessen, D., Hübner, S., Titgemeyer, F. & Stülke, J. (2006) Keeping signals straight in transcription regulation: specificity determinants for the interaction of a family of conserved bacterial RNA-protein couples. Nucl. Acids Res. 34: 6102-6115. PubMed
- Schilling, O., Langbein, I., Müller, M., Schmalisch, M. & Stülke, J. (2004) A protein-dependent riboswitch controlling ptsGHI operon expression in Bacillus subtilis: RNA structure rather than sequence provides interaction specificity. Nucl. Acids Res. 32: 2853-2864. PubMed
- Schmalisch, M., Bachem, S. & Stülke, J. (2003) Control of the Bacillus subtilis antiterminator protein GlcT by phosphorylation: Elucidation of the phosphorylation chain leading to inactivation of GlcT. J. Biol. Chem. 278: 51108-51115. PubMed