Difference between revisions of "PrpC"
| Line 82: | Line 82: | ||
* '''Structure:''' | * '''Structure:''' | ||
| − | * '''Swiss prot entry:''' | + | * '''Swiss prot entry:''' [http://www.uniprot.org/uniprot/O34779 O34779] |
* '''KEGG entry:''' [http://www.genome.jp/dbget-bin/www_bget?bsu+BSU15760] | * '''KEGG entry:''' [http://www.genome.jp/dbget-bin/www_bget?bsu+BSU15760] | ||
Revision as of 22:24, 4 May 2009
- Description: protein phosphatase
| Gene name | prpC |
| Synonyms | yloO |
| Essential | no |
| Product | protein phosphatase |
| Function | antagonist of PrkC-dependent phosphorylation |
| MW, pI | 27 kDa, 4.355 |
| Gene length, protein length | 762 bp, 254 aa |
| Immediate neighbours | yloN, prkC |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 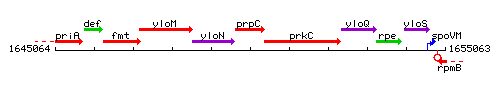
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Coordinates:
Phenotypes of a mutant
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family:
- Paralogous protein(s):
Proteins dephosphorylated by PrpC
CpgA, EF-Tu, YezB PubMed, HPr PubMed
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Interactions:
- Localization:
Database entries
- Structure:
- Swiss prot entry: O34779
- KEGG entry: [2]
- E.C. number:
Additional information
Expression and regulation
- Operon:
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
- Obuchowski, M., Madec, E., Delattre, D., Boël, G., Iwanicki, A., Foulger, D. and Séror, S. J. 2000. Characterization of PrpC from Bacillus subtilis, a member of the PPM phosphatase family. J. Bacteriol. 182: 5634-5638. PubMed
- Gaidenko TA, Kim TJ, Price CW: (2002) The PrpC serine-threonine phosphatase and PrkC kinase have opposing physiological roles in stationary-phase Bacillus subtilis cells. J Bacteriol, 184:6109-6114. PubMed
- Singh, K. D., Schmalisch, M. H., Stülke, J. & Görke, B. (2008) Carbon catabolite repression in Bacillus subtilis: A quantitative analysis of repression exerted by different carbon sources. J. Bacteriol. 190: 7275-7284. PubMed
- Absalon C, Obuchowski M, Madec E, Delattre D, Holland IB, Séror SJ (2009) CpgA, EF-Tu and the stressosome protein YezB are substrates of the Ser/Thr kinase/phosphatase couple, PrkC/PrpC, in Bacillus subtilis. Microbiology 155: 932-943. PubMed
- Author1, Author2 & Author3 (year) Title Journal volume: page-page. PubMed