PpiB
- Description: peptidyl-prolyl isomerase
| Gene name | ppiB |
| Synonyms | cypBS |
| Essential | no |
| Product | peptidyl-prolyl isomerase |
| Function | protein folding |
| Gene expression levels in SubtiExpress: ppiB | |
| MW, pI | 15 kDa, 5.472 |
| Gene length, protein length | 429 bp, 143 aa |
| Immediate neighbours | ypzD, ypuA |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 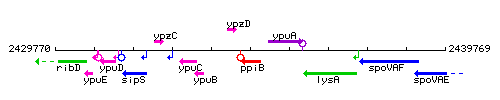
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed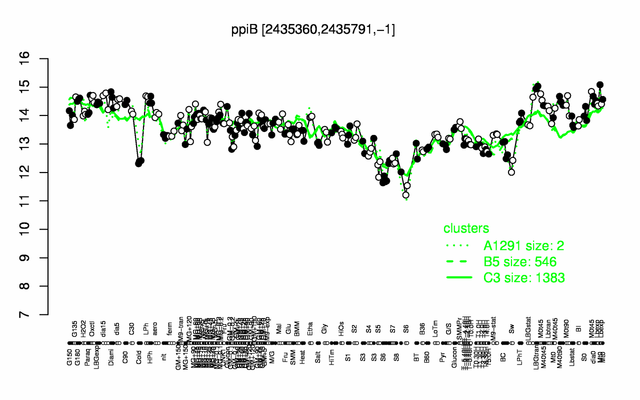
| |
Contents
Categories containing this gene/protein
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU23360
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: Peptidylproline (omega=180) = peptidylproline (omega=0) (according to Swiss-Prot)
- Protein family: cyclophilin-type PPIase family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- Structure:
- UniProt: P35137
- KEGG entry: [3]
- E.C. number: 5.2.1.8
Additional information
Expression and regulation
- Operon: ppiB PubMed
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
S F Göthel, C Scholz, F X Schmid, M A Marahiel
Cyclophilin and trigger factor from Bacillus subtilis catalyze in vitro protein folding and are necessary for viability under starvation conditions.
Biochemistry: 1998, 37(38);13392-9
[PubMed:9748346]
[WorldCat.org]
[DOI]
(P p)
T V Achenbach, S F Göthel, M A Marahiel
Histidine 109 in peptidyl-prolyl cis-trans isomerase of Bacillus subtilis plays an important role in catalysis and in cyclosporin A binding.
FEMS Microbiol Lett: 1997, 154(1);139-44
[PubMed:9297832]
[WorldCat.org]
[DOI]
(P p)
S F Göthel, M Herrler, M A Marahiel
Peptidyl-prolyl cis-trans isomerase of Bacillus subtilis: identification of residues involved in cyclosporin A affinity and catalytic efficiency.
Biochemistry: 1996, 35(11);3636-40
[PubMed:8639516]
[WorldCat.org]
[DOI]
(P p)
M Herrler, H Bang, M A Marahiel
Cloning and characterization of ppiB, a Bacillus subtilis gene which encodes a cyclosporin A-sensitive peptidyl-prolyl cis-trans isomerase.
Mol Microbiol: 1994, 11(6);1073-83
[PubMed:8022278]
[WorldCat.org]
[DOI]
(P p)
M Herrler, H Bang, K Brune, G Fischer, M A Marahiel
Peptidyl-prolyl cis-trans isomerase from Bacillus subtilis. A prokaryotic enzyme that is highly sensitive to cyclosporin A.
FEBS Lett: 1992, 309(3);231-4
[PubMed:1516692]
[WorldCat.org]
[DOI]
(P p)