Difference between revisions of "PhoR"
(→The protein) |
(→References) |
||
| Line 157: | Line 157: | ||
=References= | =References= | ||
| − | + | == Reviews == | |
| − | <pubmed>20008068 10433720,15205429,9084179,17085571,9987123,10913081 | + | <pubmed> 10094672, 25355628 </pubmed> |
| + | == Original publications == | ||
| + | <pubmed>20008068 10433720,15205429,9084179,17085571,9987123,10913081,16452408 20167622 ,15205429,14762014, 20382764 25315493</pubmed> | ||
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 12:20, 31 October 2014
- Description: two-component sensor kinase, regulation of phosphate metabolism
| Gene name | phoR |
| Synonyms | |
| Essential | no |
| Product | two-component sensor kinase |
| Function | regulation of phosphate metabolism |
| Gene expression levels in SubtiExpress: phoR | |
| Interactions involving this protein in SubtInteract: PhoR | |
| MW, pI | 64 kDa, 5.957 |
| Gene length, protein length | 1737 bp, 579 aa |
| Immediate neighbours | polA, phoP |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 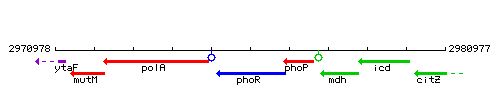
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed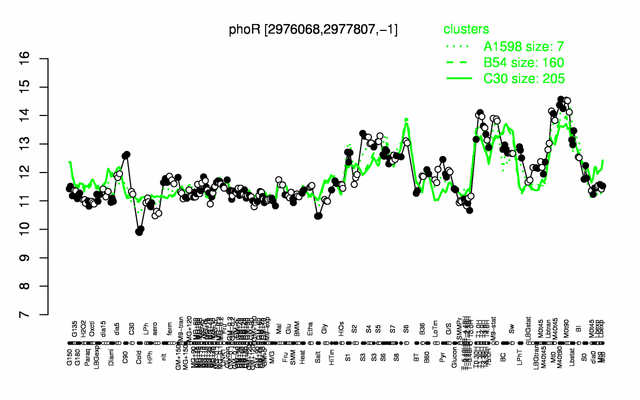
| |
Contents
Categories containing this gene/protein
phosphate metabolism, protein modification, transcription factors and their control, sporulation proteins, general stress proteins (controlled by SigB), membrane proteins, phosphoproteins
This gene is a member of the following regulons
CcpA regulon, PhoP regulon, SigB regulon, SigE regulon
The gene
Basic information
- Locus tag: BSU29100
Phenotypes of a mutant
Database entries
- BsubCyc: BSU29100
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: autophosphorylation, phosphorylation of PhoP
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- two transmembrane segments
- PAS domain, for binding of an intermediate of wall teichoic acid biosynthesis PubMed
- C-terminal histidine phosphotransferase domain
- Modification:
- autophosphorylation on a His residue in response to the the availability of an intermediate of wall teichoic acid bioynthesis, autophosphorylation is prevented by binding of this intermediate to the intracellular PAS domain of PhoR PubMed
- Cofactor(s):
- Effectors of protein activity:
- activity is inhibited by binding of an intermediate of wall teichoic acid biosynthesis to the intracellular PAS domain of PhoR PubMed
Database entries
- BsubCyc: BSU29100
- UniProt: P23545
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
- Additional information:
- number of protein molecules per cell (complex medium with amino acids, without glucose): 453 PubMed
Biological materials
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Marion Hulett, University of Illinois at Chicago, USA Homepage
Your additional remarks
References
Reviews
Georg Fritz, Thorsten Mascher
A balancing act times two: sensing and regulating cell envelope homeostasis in Bacillus subtilis.
Mol Microbiol: 2014, 94(6);1201-7
[PubMed:25355628]
[WorldCat.org]
[DOI]
(I p)
C Fabret, V A Feher, J A Hoch
Two-component signal transduction in Bacillus subtilis: how one organism sees its world.
J Bacteriol: 1999, 181(7);1975-83
[PubMed:10094672]
[WorldCat.org]
[DOI]
(P p)
Original publications
Eric Botella, Susanne Krogh Devine, Sebastian Hubner, Letal I Salzberg, Robert T Gale, Eric D Brown, Hannes Link, Uwe Sauer, Jeroen D Codée, David Noone, Kevin M Devine
PhoR autokinase activity is controlled by an intermediate in wall teichoic acid metabolism that is sensed by the intracellular PAS domain during the PhoPR-mediated phosphate limitation response of Bacillus subtilis.
Mol Microbiol: 2014, 94(6);1242-59
[PubMed:25315493]
[WorldCat.org]
[DOI]
(I p)
Bindiya Kaushal, Salbi Paul, F Marion Hulett
Direct regulation of Bacillus subtilis phoPR transcription by transition state regulator ScoC.
J Bacteriol: 2010, 192(12);3103-13
[PubMed:20382764]
[WorldCat.org]
[DOI]
(I p)
Inga Jende, Kottayil I Varughese, Kevin M Devine
Amino acid identity at one position within the alpha1 helix of both the histidine kinase and the response regulator of the WalRK and PhoPR two-component systems plays a crucial role in the specificity of phosphotransfer.
Microbiology (Reading): 2010, 156(Pt 6);1848-1859
[PubMed:20167622]
[WorldCat.org]
[DOI]
(I p)
Changsoo Chang, Christine Tesar, Minyi Gu, Gyorgy Babnigg, Andrzej Joachimiak, P Raj Pokkuluri, Hendrik Szurmant, Marianne Schiffer
Extracytoplasmic PAS-like domains are common in signal transduction proteins.
J Bacteriol: 2010, 192(4);1156-9
[PubMed:20008068]
[WorldCat.org]
[DOI]
(I p)
Amr Eldakak, F Marion Hulett
Cys303 in the histidine kinase PhoR is crucial for the phosphotransfer reaction in the PhoPR two-component system in Bacillus subtilis.
J Bacteriol: 2007, 189(2);410-21
[PubMed:17085571]
[WorldCat.org]
[DOI]
(P p)
Ankita Puri-Taneja, Salbi Paul, Yinghua Chen, F Marion Hulett
CcpA causes repression of the phoPR promoter through a novel transcription start site, P(A6).
J Bacteriol: 2006, 188(4);1266-78
[PubMed:16452408]
[WorldCat.org]
[DOI]
(P p)
Salbi Paul, Stephanie Birkey, Wei Liu, F Marion Hulett
Autoinduction of Bacillus subtilis phoPR operon transcription results from enhanced transcription from EsigmaA- and EsigmaE-responsive promoters by phosphorylated PhoP.
J Bacteriol: 2004, 186(13);4262-75
[PubMed:15205429]
[WorldCat.org]
[DOI]
(P p)
Zoltán Prágai, Nicholas E E Allenby, Nicola O'Connor, Sarah Dubrac, Georges Rapoport, Tarek Msadek, Colin R Harwood
Transcriptional regulation of the phoPR operon in Bacillus subtilis.
J Bacteriol: 2004, 186(4);1182-90
[PubMed:14762014]
[WorldCat.org]
[DOI]
(P p)
H Antelmann, C Scharf, M Hecker
Phosphate starvation-inducible proteins of Bacillus subtilis: proteomics and transcriptional analysis.
J Bacteriol: 2000, 182(16);4478-90
[PubMed:10913081]
[WorldCat.org]
[DOI]
(P p)
L Shi, W Liu, F M Hulett
Decay of activated Bacillus subtilis pho response regulator, PhoP approximately P, involves the PhoR approximately P intermediate.
Biochemistry: 1999, 38(31);10119-25
[PubMed:10433720]
[WorldCat.org]
[DOI]
(P p)
L Shi, F M Hulett
The cytoplasmic kinase domain of PhoR is sufficient for the low phosphate-inducible expression of pho regulon genes in Bacillus subtilis.
Mol Microbiol: 1999, 31(1);211-22
[PubMed:9987123]
[WorldCat.org]
[DOI]
(P p)
Jörg P Müler, Zhidong An, Tarek Merad, Ian C Hancock, Colin R Harwood
Influence of Bacillus subtilis phoR on cell wall anionic polymers.
Microbiology (Reading): 1997, 143 ( Pt 3);947-956
[PubMed:9084179]
[WorldCat.org]
[DOI]
(P p)