Difference between revisions of "PhoR"
| Line 135: | Line 135: | ||
* '''Additional information:''' | * '''Additional information:''' | ||
| + | ** number of protein molecules per cell (complex medium with amino acids, without glucose): 453 {{PubMed|24696501}} | ||
=Biological materials = | =Biological materials = | ||
Revision as of 10:09, 17 April 2014
- Description: two-component sensor kinase, regulation of phosphate metabolism
| Gene name | phoR |
| Synonyms | |
| Essential | no |
| Product | two-component sensor kinase |
| Function | regulation of phosphate metabolism |
| Gene expression levels in SubtiExpress: phoR | |
| Interactions involving this protein in SubtInteract: PhoR | |
| MW, pI | 64 kDa, 5.957 |
| Gene length, protein length | 1737 bp, 579 aa |
| Immediate neighbours | polA, phoP |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 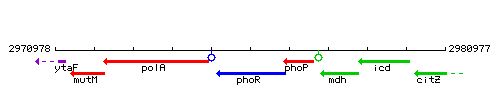
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed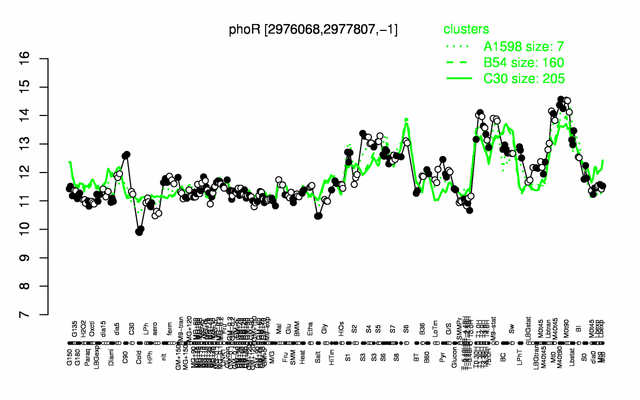
| |
Contents
Categories containing this gene/protein
phosphate metabolism, protein modification, transcription factors and their control, sporulation proteins, general stress proteins (controlled by SigB), membrane proteins, phosphoproteins
This gene is a member of the following regulons
CcpA regulon, PhoP regulon, SigB regulon, SigE regulon
The gene
Basic information
- Locus tag: BSU29100
Phenotypes of a mutant
Database entries
- BsubCyc: BSU29100
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: autophosphorylation, phosphorylation of PhoP
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains: two transmembrane segments, C-terminal histidine phosphotransferase domain
- Modification: autophosphorylation on a His residue
- Cofactor(s):
- Effectors of protein activity:
Database entries
- BsubCyc: BSU29100
- UniProt: P23545
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
- Additional information:
- number of protein molecules per cell (complex medium with amino acids, without glucose): 453 PubMed
Biological materials
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Marion Hulett, University of Illinois at Chicago, USA Homepage
Your additional remarks
References
Bindiya Kaushal, Salbi Paul, F Marion Hulett
Direct regulation of Bacillus subtilis phoPR transcription by transition state regulator ScoC.
J Bacteriol: 2010, 192(12);3103-13
[PubMed:20382764]
[WorldCat.org]
[DOI]
(I p)
Inga Jende, Kottayil I Varughese, Kevin M Devine
Amino acid identity at one position within the alpha1 helix of both the histidine kinase and the response regulator of the WalRK and PhoPR two-component systems plays a crucial role in the specificity of phosphotransfer.
Microbiology (Reading): 2010, 156(Pt 6);1848-1859
[PubMed:20167622]
[WorldCat.org]
[DOI]
(I p)
Changsoo Chang, Christine Tesar, Minyi Gu, Gyorgy Babnigg, Andrzej Joachimiak, P Raj Pokkuluri, Hendrik Szurmant, Marianne Schiffer
Extracytoplasmic PAS-like domains are common in signal transduction proteins.
J Bacteriol: 2010, 192(4);1156-9
[PubMed:20008068]
[WorldCat.org]
[DOI]
(I p)
Amr Eldakak, F Marion Hulett
Cys303 in the histidine kinase PhoR is crucial for the phosphotransfer reaction in the PhoPR two-component system in Bacillus subtilis.
J Bacteriol: 2007, 189(2);410-21
[PubMed:17085571]
[WorldCat.org]
[DOI]
(P p)
Ankita Puri-Taneja, Salbi Paul, Yinghua Chen, F Marion Hulett
CcpA causes repression of the phoPR promoter through a novel transcription start site, P(A6).
J Bacteriol: 2006, 188(4);1266-78
[PubMed:16452408]
[WorldCat.org]
[DOI]
(P p)
Salbi Paul, Stephanie Birkey, Wei Liu, F Marion Hulett
Autoinduction of Bacillus subtilis phoPR operon transcription results from enhanced transcription from EsigmaA- and EsigmaE-responsive promoters by phosphorylated PhoP.
J Bacteriol: 2004, 186(13);4262-75
[PubMed:15205429]
[WorldCat.org]
[DOI]
(P p)
Zoltán Prágai, Nicholas E E Allenby, Nicola O'Connor, Sarah Dubrac, Georges Rapoport, Tarek Msadek, Colin R Harwood
Transcriptional regulation of the phoPR operon in Bacillus subtilis.
J Bacteriol: 2004, 186(4);1182-90
[PubMed:14762014]
[WorldCat.org]
[DOI]
(P p)
H Antelmann, C Scharf, M Hecker
Phosphate starvation-inducible proteins of Bacillus subtilis: proteomics and transcriptional analysis.
J Bacteriol: 2000, 182(16);4478-90
[PubMed:10913081]
[WorldCat.org]
[DOI]
(P p)
L Shi, W Liu, F M Hulett
Decay of activated Bacillus subtilis pho response regulator, PhoP approximately P, involves the PhoR approximately P intermediate.
Biochemistry: 1999, 38(31);10119-25
[PubMed:10433720]
[WorldCat.org]
[DOI]
(P p)
C Fabret, V A Feher, J A Hoch
Two-component signal transduction in Bacillus subtilis: how one organism sees its world.
J Bacteriol: 1999, 181(7);1975-83
[PubMed:10094672]
[WorldCat.org]
[DOI]
(P p)
L Shi, F M Hulett
The cytoplasmic kinase domain of PhoR is sufficient for the low phosphate-inducible expression of pho regulon genes in Bacillus subtilis.
Mol Microbiol: 1999, 31(1);211-22
[PubMed:9987123]
[WorldCat.org]
[DOI]
(P p)
Jörg P Müler, Zhidong An, Tarek Merad, Ian C Hancock, Colin R Harwood
Influence of Bacillus subtilis phoR on cell wall anionic polymers.
Microbiology (Reading): 1997, 143 ( Pt 3);947-956
[PubMed:9084179]
[WorldCat.org]
[DOI]
(P p)