PhoD
- Description: phosphodiesterase/alkaline phosphatase
| Gene name | phoD |
| Synonyms | ycbS |
| Essential | no |
| Product | phosphodiesterase/alkaline phosphatase |
| Function | aquisition of phosphate upon phosphoate starvation |
| Gene expression levels in SubtiExpress: phoD | |
| Interactions involving this protein in SubtInteract: PhoD | |
| Metabolic function and regulation of this protein in SubtiPathways: Protein secretion | |
| MW, pI | 62 kDa, 8.394 |
| Gene length, protein length | 1668 bp, 556 aa |
| Immediate neighbours | yczK, tatAD |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 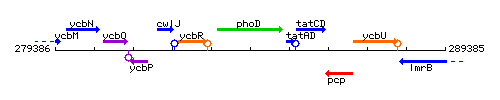
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed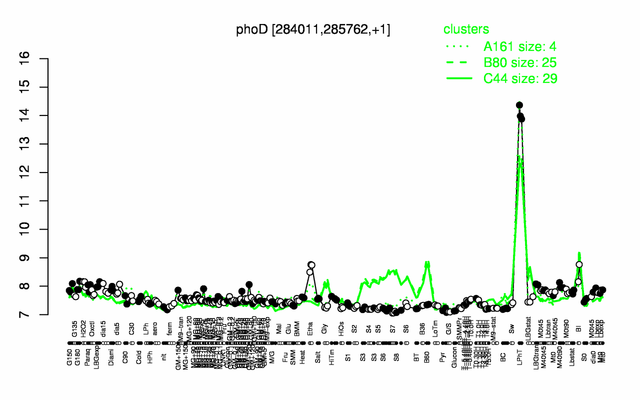
| |
Contents
Categories containing this gene/protein
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU02620
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: A phosphate monoester + H2O = an alcohol + phosphate (according to Swiss-Prot)
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization: extracellular (signal peptide) PubMed, secreted by the TatAD-TatCD complex PubMed
Database entries
- Structure: 2YEQ
- UniProt: P42251
- KEGG entry: [3]
- E.C. number: 3.1.3.1
Additional information
Expression and regulation
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Oscar Kuipers, University of Groningen, The Netherlands Homepage
Your additional remarks
References
Additional publications: PubMed
Pierre Nicolas, Ulrike Mäder, Etienne Dervyn, Tatiana Rochat, Aurélie Leduc, Nathalie Pigeonneau, Elena Bidnenko, Elodie Marchadier, Mark Hoebeke, Stéphane Aymerich, Dörte Becher, Paola Bisicchia, Eric Botella, Olivier Delumeau, Geoff Doherty, Emma L Denham, Mark J Fogg, Vincent Fromion, Anne Goelzer, Annette Hansen, Elisabeth Härtig, Colin R Harwood, Georg Homuth, Hanne Jarmer, Matthieu Jules, Edda Klipp, Ludovic Le Chat, François Lecointe, Peter Lewis, Wolfram Liebermeister, Anika March, Ruben A T Mars, Priyanka Nannapaneni, David Noone, Susanne Pohl, Bernd Rinn, Frank Rügheimer, Praveen K Sappa, Franck Samson, Marc Schaffer, Benno Schwikowski, Leif Steil, Jörg Stülke, Thomas Wiegert, Kevin M Devine, Anthony J Wilkinson, Jan Maarten van Dijl, Michael Hecker, Uwe Völker, Philippe Bessières, Philippe Noirot
Condition-dependent transcriptome reveals high-level regulatory architecture in Bacillus subtilis.
Science: 2012, 335(6072);1103-6
[PubMed:22383849]
[WorldCat.org]
[DOI]
(I p)
Anja N J A Ridder, Esther J de Jong, Jan D H Jongbloed, Oscar P Kuipers
Subcellular localization of TatAd of Bacillus subtilis depends on the presence of TatCd or TatCy.
J Bacteriol: 2009, 191(13);4410-8
[PubMed:19395490]
[WorldCat.org]
[DOI]
(I p)
Robyn T Eijlander, Magdalena A Kolbusz, Erwin M Berendsen, Oscar P Kuipers
Effects of altered TatC proteins on protein secretion efficiency via the twin-arginine translocation pathway of Bacillus subtilis.
Microbiology (Reading): 2009, 155(Pt 6);1776-1785
[PubMed:19383693]
[WorldCat.org]
[DOI]
(P p)
Thijs R H M Kouwen, René van der Ploeg, Haike Antelmann, Michael Hecker, Georg Homuth, Ulrike Mäder, Jan Maarten van Dijl
Overflow of a hyper-produced secretory protein from the Bacillus Sec pathway into the Tat pathway for protein secretion as revealed by proteogenomics.
Proteomics: 2009, 9(4);1018-32
[PubMed:19180538]
[WorldCat.org]
[DOI]
(I p)
Birgit Voigt, Haike Antelmann, Dirk Albrecht, Armin Ehrenreich, Karl-Heinz Maurer, Stefan Evers, Gerhard Gottschalk, Jan Maarten van Dijl, Thomas Schweder, Michael Hecker
Cell physiology and protein secretion of Bacillus licheniformis compared to Bacillus subtilis.
J Mol Microbiol Biotechnol: 2009, 16(1-2);53-68
[PubMed:18957862]
[WorldCat.org]
[DOI]
(I p)
Ovidiu I Pop, Martin Westermann, Rudolf Volkmer-Engert, Daniela Schulz, Cornelius Lemke, Sandra Schreiber, Roman Gerlach, Reinhard Wetzker, Jörg P Müller
Sequence-specific binding of prePhoD to soluble TatAd indicates protein-mediated targeting of the Tat export in Bacillus subtilis.
J Biol Chem: 2003, 278(40);38428-36
[PubMed:12867413]
[WorldCat.org]
[DOI]
(P p)
Jan D H Jongbloed, Haike Antelmann, Michael Hecker, Reindert Nijland, Sierd Bron, Ulla Airaksinen, Frens Pries, Wim J Quax, Jan Maarten van Dijl, Peter G Braun
Selective contribution of the twin-arginine translocation pathway to protein secretion in Bacillus subtilis.
J Biol Chem: 2002, 277(46);44068-78
[PubMed:12218047]
[WorldCat.org]
[DOI]
(P p)
H Antelmann, C Scharf, M Hecker
Phosphate starvation-inducible proteins of Bacillus subtilis: proteomics and transcriptional analysis.
J Bacteriol: 2000, 182(16);4478-90
[PubMed:10913081]
[WorldCat.org]
[DOI]
(P p)
J P Müller, M Wagner
Localisation of the cell wall-associated phosphodiesterase PhoD of Bacillus subtilis.
FEMS Microbiol Lett: 1999, 180(2);287-96
[PubMed:10556724]
[WorldCat.org]
[DOI]
(P p)
S Eder, W Liu, F M Hulett
Mutational analysis of the phoD promoter in Bacillus subtilis: implications for PhoP binding and promoter activation of Pho regulon promoters.
J Bacteriol: 1999, 181(7);2017-25
[PubMed:10094677]
[WorldCat.org]
[DOI]
(P p)
S Eder, L Shi, K Jensen, K Yamane, F M Hulett
A Bacillus subtilis secreted phosphodiesterase/alkaline phosphatase is the product of a Pho regulon gene, phoD.
Microbiology (Reading): 1996, 142 ( Pt 8);2041-7
[PubMed:8760916]
[WorldCat.org]
[DOI]
(P p)