Pgi
- Description: glucose 6-phosphate isomerase, glycolytic / gluconeogenic enzyme
| Gene name | pgi |
| Synonyms | yugL |
| Essential | no |
| Product | glucose-6-phosphate isomerase |
| Function | enzyme in glycolysis / gluconeogenesis |
| Gene expression levels in SubtiExpress: pgi | |
| Metabolic function and regulation of this protein in SubtiPathways: pgi | |
| MW, pI | 50.4 kDa, 4.85 |
| Gene length, protein length | 1353 bp, 451 amino acids |
| Immediate neighbours | yugM, yugK |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 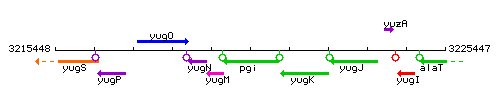
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed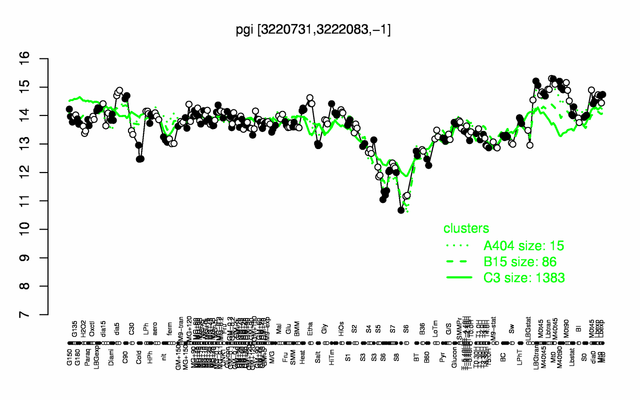
| |
Contents
Categories containing this gene/protein
carbon core metabolism, phosphoproteins, most abundant proteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU31350
Phenotypes of a mutant
Database entries
- BsubCyc: BSU31350
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: D-glucose 6-phosphate = D-fructose 6-phosphate (according to Swiss-Prot) D-glucose 6-phosphate = D-fructose 6-phosphate
- Protein family: GPI family (according to Swiss-Prot) GPI family
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Modification: phosphorylation on Thr-39 PubMed
- Effectors of protein activity: competitively inhibited by 6-phosphogluconate (in B.caldotenax, B.stearothermophilus) PubMed
- Localization:
- cytoplasm (according to Swiss-Prot), cytoplasm
Database entries
- BsubCyc: BSU31350
- UniProt: P80860
- KEGG entry: [3]
- E.C. number: 5.3.1.9
Additional information
- extensive information on the structure and enzymatic properties of Pgi can be found at Proteopedia
Expression and regulation
- Regulation: constitutively expressed PubMed
- Additional information:
- belongs to the 100 most abundant proteins PubMed
Biological materials
- Mutant: GP508 (spc), available in Jörg Stülke's lab, PubMed
- Expression vector:
- pGP398 (N-terminal His-tag, in pWH844), available in Jörg Stülke's lab
- lacZ fusion: pGP510 (in pAC6), available in Jörg Stülke's lab
- GFP fusion:
- two-hybrid system: B. pertussis adenylate cyclase-based bacterial two hybrid system (BACTH), available in Jörg Stülke's lab
- Antibody:
Labs working on this gene/protein
Jörg Stülke, University of Göttingen, Germany homepage
Your additional remarks
References
Michael Kohlstedt, Praveen K Sappa, Hanna Meyer, Sandra Maaß, Adrienne Zaprasis, Tamara Hoffmann, Judith Becker, Leif Steil, Michael Hecker, Jan Maarten van Dijl, Michael Lalk, Ulrike Mäder, Jörg Stülke, Erhard Bremer, Uwe Völker, Christoph Wittmann
Adaptation of Bacillus subtilis carbon core metabolism to simultaneous nutrient limitation and osmotic challenge: a multi-omics perspective.
Environ Microbiol: 2014, 16(6);1898-917
[PubMed:24571712]
[WorldCat.org]
[DOI]
(I p)
Fabian M Commichau, Nico Pietack, Jörg Stülke
Essential genes in Bacillus subtilis: a re-evaluation after ten years.
Mol Biosyst: 2013, 9(6);1068-75
[PubMed:23420519]
[WorldCat.org]
[DOI]
(I p)
Yian Liang Lee, TienHsiung Thomas Li
Crystallization and preliminary crystallographic study of the phosphoglucose isomerase from Bacillus subtilis.
Acta Crystallogr Sect F Struct Biol Cryst Commun: 2008, 64(Pt 12);1181-3
[PubMed:19052382]
[WorldCat.org]
[DOI]
(I p)
Boris Macek, Ivan Mijakovic, Jesper V Olsen, Florian Gnad, Chanchal Kumar, Peter R Jensen, Matthias Mann
The serine/threonine/tyrosine phosphoproteome of the model bacterium Bacillus subtilis.
Mol Cell Proteomics: 2007, 6(4);697-707
[PubMed:17218307]
[WorldCat.org]
[DOI]
(P p)
Christine Eymann, Annette Dreisbach, Dirk Albrecht, Jörg Bernhardt, Dörte Becher, Sandy Gentner, Le Thi Tam, Knut Büttner, Gerrit Buurman, Christian Scharf, Simone Venz, Uwe Völker, Michael Hecker
A comprehensive proteome map of growing Bacillus subtilis cells.
Proteomics: 2004, 4(10);2849-76
[PubMed:15378759]
[WorldCat.org]
[DOI]
(P p)
J Stülke, I Martin-Verstraete, P Glaser, G Rapoport
Characterization of glucose-repression-resistant mutants of Bacillus subtilis: identification of the glcR gene.
Arch Microbiol: 2001, 175(6);441-9
[PubMed:11491085]
[WorldCat.org]
[DOI]
(P p)
H Ludwig, G Homuth, M Schmalisch, F M Dyka, M Hecker, J Stülke
Transcription of glycolytic genes and operons in Bacillus subtilis: evidence for the presence of multiple levels of control of the gapA operon.
Mol Microbiol: 2001, 41(2);409-22
[PubMed:11489127]
[WorldCat.org]
[DOI]
(P p)
J Y Lin, C Prasad
Selection of a mutant of Bacillus subtilis deficient in glucose-6-phosphate dehydrogenase and phosphoglucoisomerase.
J Gen Microbiol: 1974, 83(2);419-21
[PubMed:4214896]
[WorldCat.org]
[DOI]
(P p)
C Prasad, E Freese
Cell lysis of Bacillus subtilis caused by intracellular accumulation of glucose-1-phosphate.
J Bacteriol: 1974, 118(3);1111-22
[PubMed:4275311]
[WorldCat.org]
[DOI]
(P p)