Difference between revisions of "Pgi"
| Line 120: | Line 120: | ||
=References= | =References= | ||
| + | <pubmed>17218307 19052382 4214896 11489127 4275311 11491085 17218307, </pubmed> | ||
# Macek et al. (2007) The serine/ threonine/ tyrosine phosphoproteome of the model bacterium ''Bacillus subtilis''. Mol. Cell. Proteomics 6: 697-707 [http://www.ncbi.nlm.nih.gov/pubmed/17218307 PubMed] | # Macek et al. (2007) The serine/ threonine/ tyrosine phosphoproteome of the model bacterium ''Bacillus subtilis''. Mol. Cell. Proteomics 6: 697-707 [http://www.ncbi.nlm.nih.gov/pubmed/17218307 PubMed] | ||
# Lee et al. (2008) Crystallization and preliminary crystallographic study of the phosphoglucose isomerase from Bacillus subtilis. Acta Cryst. Sect. F 64:1181-1183. [http://www.ncbi.nlm.nih.gov/sites/entrez/19052382 PubMed] | # Lee et al. (2008) Crystallization and preliminary crystallographic study of the phosphoglucose isomerase from Bacillus subtilis. Acta Cryst. Sect. F 64:1181-1183. [http://www.ncbi.nlm.nih.gov/sites/entrez/19052382 PubMed] | ||
Revision as of 21:12, 8 June 2009
- Description: glucose 6-phosphate isomerase, glycolytic / gluconeogenic enzyme
| Gene name | pgi |
| Synonyms | yugL |
| Essential | no |
| Product | glucose-6-phosphate isomerase |
| Function | enzyme in glycolysis / gluconeogenesis |
| MW, pI | 50.4 kDa, 4.85 |
| Gene length, protein length | 1353 bp, 451 amino acids |
| Immediate neighbours | yugM, yugK |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 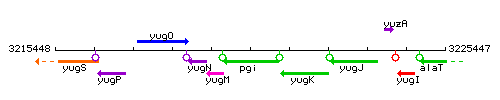
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU31350
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: D-glucose 6-phosphate = D-fructose 6-phosphate (according to Swiss-Prot) D-glucose 6-phosphate = D-fructose 6-phosphate
- Protein family: GPI family (according to Swiss-Prot) GPI family
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification: phosphorylation on Thr-39 PubMed
- Cofactor(s):
- Effectors of protein activity: inhibited by 6-phosphogluconate (in B.caldotenax, B.stearothermophilus) PubMed
- Interactions:
- Localization: cytoplasm (according to Swiss-Prot), cytoplasm
Database entries
- Swiss prot entry: P80860
- KEGG entry: [3]
- E.C. number: 5.3.1.9
Additional information
Expression and regulation
- Operon: pgi PubMed
- Sigma factor:
- Regulation: constitutively expressed PubMed
- Additional information:
Biological materials
- Mutant: GP508 (spc), available in Stülke lab
- GFP fusion:
- two-hybrid system: B. pertussis adenylate cyclase-based bacterial two hybrid system (BACTH), available in Stülke lab
- Antibody:
Labs working on this gene/protein
Jörg Stülke, University of Göttingen, Germany homepage
Your additional remarks
References
Yian Liang Lee, TienHsiung Thomas Li
Crystallization and preliminary crystallographic study of the phosphoglucose isomerase from Bacillus subtilis.
Acta Crystallogr Sect F Struct Biol Cryst Commun: 2008, 64(Pt 12);1181-3
[PubMed:19052382]
[WorldCat.org]
[DOI]
(I p)
Boris Macek, Ivan Mijakovic, Jesper V Olsen, Florian Gnad, Chanchal Kumar, Peter R Jensen, Matthias Mann
The serine/threonine/tyrosine phosphoproteome of the model bacterium Bacillus subtilis.
Mol Cell Proteomics: 2007, 6(4);697-707
[PubMed:17218307]
[WorldCat.org]
[DOI]
(P p)
J Stülke, I Martin-Verstraete, P Glaser, G Rapoport
Characterization of glucose-repression-resistant mutants of Bacillus subtilis: identification of the glcR gene.
Arch Microbiol: 2001, 175(6);441-9
[PubMed:11491085]
[WorldCat.org]
[DOI]
(P p)
H Ludwig, G Homuth, M Schmalisch, F M Dyka, M Hecker, J Stülke
Transcription of glycolytic genes and operons in Bacillus subtilis: evidence for the presence of multiple levels of control of the gapA operon.
Mol Microbiol: 2001, 41(2);409-22
[PubMed:11489127]
[WorldCat.org]
[DOI]
(P p)
J Y Lin, C Prasad
Selection of a mutant of Bacillus subtilis deficient in glucose-6-phosphate dehydrogenase and phosphoglucoisomerase.
J Gen Microbiol: 1974, 83(2);419-21
[PubMed:4214896]
[WorldCat.org]
[DOI]
(P p)
C Prasad, E Freese
Cell lysis of Bacillus subtilis caused by intracellular accumulation of glucose-1-phosphate.
J Bacteriol: 1974, 118(3);1111-22
[PubMed:4275311]
[WorldCat.org]
[DOI]
(P p)
- Macek et al. (2007) The serine/ threonine/ tyrosine phosphoproteome of the model bacterium Bacillus subtilis. Mol. Cell. Proteomics 6: 697-707 PubMed
- Lee et al. (2008) Crystallization and preliminary crystallographic study of the phosphoglucose isomerase from Bacillus subtilis. Acta Cryst. Sect. F 64:1181-1183. PubMed
- Lin and Prasad (1975) Selection of a mutant of Bacillus subtilis deficient in glucose-6-phosphate dehydrogenase and phosphoglucoisomerase. J. Gen. Microbiol. 83:419-421. PubMed
- Ludwig, H., Homuth, G., Schmalisch, M., Dyka, F. M., Hecker, M., and Stülke, J. (2001) Transcription of glycolytic genes and operons in Bacillus subtilis: evidence for the presence of multiple levels of control of the gapA operon. Mol Microbiol 41, 409-422.PubMed
- Prasad and Freese (1974) Cell lysis of Bacillus subtilis caused by intracellular accumulation of glucose-1-phosphate. J. Bacteriol. 118:1111-1122. PubMed
- Stülke, J., Martin-Verstraete, I., Glaser, P. & Rapoport, G. (2001) Characterization of glucose repression resistant mutants of Bacillus subtilis: identification of the glcR gene. Arch. Microbiol. 175: 441-449. PubMed
- Macek et al. (2007) The serine/threonine/tyrosine phosphoproteome of the model bacterium Bacillus subtilis. Mol Cell Proteomics 6(4): 697-707. PubMed