Difference between revisions of "PgcA"
| Line 38: | Line 38: | ||
<br/><br/><br/><br/> | <br/><br/><br/><br/> | ||
<br/><br/><br/><br/> | <br/><br/><br/><br/> | ||
| − | + | <br/><br/> | |
| − | |||
| − | |||
| − | |||
| − | |||
= [[Categories]] containing this gene/protein = | = [[Categories]] containing this gene/protein = | ||
| Line 59: | Line 55: | ||
===Phenotypes of a mutant === | ===Phenotypes of a mutant === | ||
| − | + | * the inactivation of ''[[pgcA]]'' suppresses the poor and filametous growth of the ''[[yvcL]] [[zapA]]'' double mutant {{PubMed|24097947}} | |
=== Database entries === | === Database entries === | ||
| Line 85: | Line 81: | ||
* '''Kinetic information:''' | * '''Kinetic information:''' | ||
| − | * '''Domains:''' | + | * '''[[Domains]]:''' |
* '''Modification:''' phosphorylated on ser/ thr/ tyr [http://www.ncbi.nlm.nih.gov/pubmed/16493705 PubMed], [http://www.ncbi.nlm.nih.gov/pubmed/17726680 PubMed] | * '''Modification:''' phosphorylated on ser/ thr/ tyr [http://www.ncbi.nlm.nih.gov/pubmed/16493705 PubMed], [http://www.ncbi.nlm.nih.gov/pubmed/17726680 PubMed] | ||
| − | * ''' | + | * '''[[Cofactors]]:''' |
* '''Effectors of protein activity:''' | * '''Effectors of protein activity:''' | ||
| Line 117: | Line 113: | ||
* '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=pgcA_1006774_1008519_1 pgcA] {{PubMed|22383849}} | * '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=pgcA_1006774_1008519_1 pgcA] {{PubMed|22383849}} | ||
| − | * '''Sigma factor:''' | + | * '''[[Sigma factor]]:''' |
* '''Regulation:''' | * '''Regulation:''' | ||
| Line 147: | Line 143: | ||
<pubmed>20735481 </pubmed> | <pubmed>20735481 </pubmed> | ||
==Original publications== | ==Original publications== | ||
| − | + | <pubmed>,15175311,17726680 16493705, 15640167 17662947 22396664 24097947</pubmed> | |
| − | <pubmed>,15175311,17726680 16493705, 15640167 17662947 </pubmed> | ||
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 19:17, 21 December 2013
- Description: alpha-phosphoglucomutase, required for UDP-glucose synthesis, inhibits FtsZ ring assembly (indirect effect due to a defect in UDP-glucose synthesis)
| Gene name | pgcA |
| Synonyms | yhxB, gtaC, gtaE |
| Essential | no |
| Product | alpha-phosphoglucomutase |
| Function | interconversion of glucose 6-phosphate and alpha-glucose 1-phosphate |
| Gene expression levels in SubtiExpress: pgcA | |
| Metabolic function and regulation of this protein in SubtiPathways: Lipid synthesis | |
| MW, pI | 62 kDa, 4.913 |
| Gene length, protein length | 1695 bp, 565 aa |
| Immediate neighbours | glpD, yhcY |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 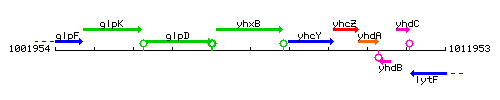
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed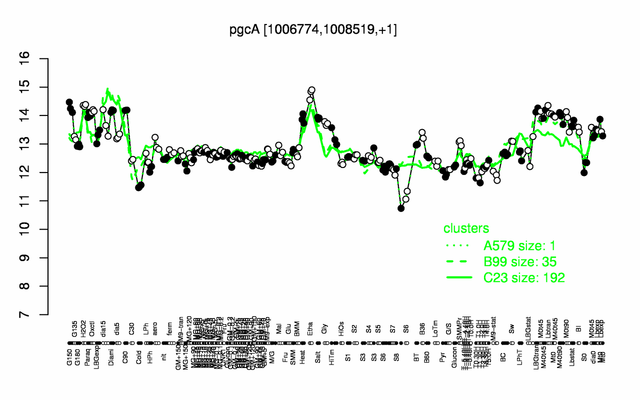
| |
Contents
Categories containing this gene/protein
cell wall synthesis, lipid metabolism/ other, phosphoproteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU09310
Phenotypes of a mutant
- the inactivation of pgcA suppresses the poor and filametous growth of the yvcL zapA double mutant PubMed
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: Alpha-D-glucose 1-phosphate = alpha-D-glucose 6-phosphate (according to Swiss-Prot)
- Protein family: phosphohexose mutase family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Effectors of protein activity:
Database entries
- Structure:
- UniProt: P18159
- KEGG entry: [2]
- E.C. number: 5.4.2.2
Additional information
PgcA inhibits FtsZ ring assembly (indirect effect due to a defect in UDP-glucose synthesis)PubMed
Expression and regulation
- Operon:
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Massimiliano Marvasi, Pieter T Visscher, Lilliam Casillas Martinez
Exopolymeric substances (EPS) from Bacillus subtilis: polymers and genes encoding their synthesis.
FEMS Microbiol Lett: 2010, 313(1);1-9
[PubMed:20735481]
[WorldCat.org]
[DOI]
(I p)
Original publications
Katarina Surdova, Pamela Gamba, Dennis Claessen, Tjalling Siersma, Martijs J Jonker, Jeff Errington, Leendert W Hamoen
The conserved DNA-binding protein WhiA is involved in cell division in Bacillus subtilis.
J Bacteriol: 2013, 195(24);5450-60
[PubMed:24097947]
[WorldCat.org]
[DOI]
(I p)
Norbert S Hill, Ryosuke Kadoya, Dhruba K Chattoraj, Petra Anne Levin
Cell size and the initiation of DNA replication in bacteria.
PLoS Genet: 2012, 8(3);e1002549
[PubMed:22396664]
[WorldCat.org]
[DOI]
(I p)
Christine Eymann, Dörte Becher, Jörg Bernhardt, Katrin Gronau, Anja Klutzny, Michael Hecker
Dynamics of protein phosphorylation on Ser/Thr/Tyr in Bacillus subtilis.
Proteomics: 2007, 7(19);3509-26
[PubMed:17726680]
[WorldCat.org]
[DOI]
(P p)
Richard B Weart, Amy H Lee, An-Chun Chien, Daniel P Haeusser, Norbert S Hill, Petra Anne Levin
A metabolic sensor governing cell size in bacteria.
Cell: 2007, 130(2);335-47
[PubMed:17662947]
[WorldCat.org]
[DOI]
(P p)
Alain Lévine, Françoise Vannier, Cédric Absalon, Lauriane Kuhn, Peter Jackson, Elaine Scrivener, Valérie Labas, Joëlle Vinh, Patrick Courtney, Jérôme Garin, Simone J Séror
Analysis of the dynamic Bacillus subtilis Ser/Thr/Tyr phosphoproteome implicated in a wide variety of cellular processes.
Proteomics: 2006, 6(7);2157-73
[PubMed:16493705]
[WorldCat.org]
[DOI]
(P p)
Vladimir Lazarevic, Blazenka Soldo, Noël Médico, Harold Pooley, Sierd Bron, Dimitri Karamata
Bacillus subtilis alpha-phosphoglucomutase is required for normal cell morphology and biofilm formation.
Appl Environ Microbiol: 2005, 71(1);39-45
[PubMed:15640167]
[WorldCat.org]
[DOI]
(P p)
Steven S Branda, José Eduardo González-Pastor, Etienne Dervyn, S Dusko Ehrlich, Richard Losick, Roberto Kolter
Genes involved in formation of structured multicellular communities by Bacillus subtilis.
J Bacteriol: 2004, 186(12);3970-9
[PubMed:15175311]
[WorldCat.org]
[DOI]
(P p)