Difference between revisions of "PfkA"
| Line 58: | Line 58: | ||
=== Database entries === | === Database entries === | ||
| + | * '''BsubCyc:''' [http://bsubcyc.org/BSUB/NEW-IMAGE?type=NIL&object=BSU29190&redirect=T BSU29190] | ||
* '''DBTBS entry:''' [http://dbtbs.hgc.jp/COG/prom/pfkA-pyk-ytzA.html] | * '''DBTBS entry:''' [http://dbtbs.hgc.jp/COG/prom/pfkA-pyk-ytzA.html] | ||
| Line 107: | Line 108: | ||
=== Database entries === | === Database entries === | ||
| + | * '''BsubCyc:''' [http://bsubcyc.org/BSUB/NEW-IMAGE?type=NIL&object=BSU29190&redirect=T BSU29190] | ||
* '''Structure:''' | * '''Structure:''' | ||
Revision as of 14:29, 2 April 2014
- Description: phosphofructokinase, glycolytic enzyme
| Gene name | pfkA |
| Synonyms | pfk |
| Essential | no |
| Product | 6-phosphofructokinase |
| Function | catabolic enzyme in glycolysis |
| Gene expression levels in SubtiExpress: pfkA | |
| Interactions involving this protein in SubtInteract: PfkA | |
| Metabolic function and regulation of this protein in SubtiPathways: pfkA | |
| MW, pI | 34,1 kDa, 6.14 |
| Gene length, protein length | 957 bp, 319 amino acids |
| Immediate neighbours | pyk, accA |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 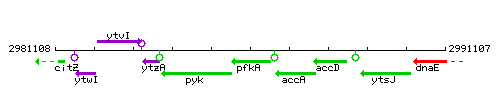
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed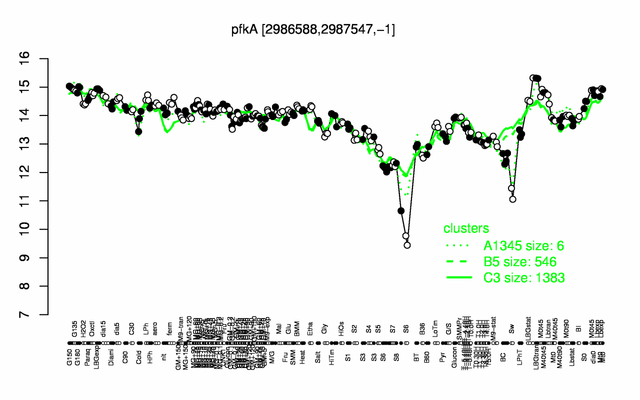
| |
Contents
Categories containing this gene/protein
carbon core metabolism, phosphoproteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU29190
Phenotypes of a mutant
Database entries
- BsubCyc: BSU29190
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: ATP + D-fructose 6-phosphate = ADP + D-fructose 1,6-bisphosphate (according to Swiss-Prot)
- Protein family: phosphofructokinase family (according to Swiss-Prot) phosphofructokinase family
- Paralogous protein(s):
Extended information on the protein
- Kinetic information: Allosteric Regulation (Reversible) PubMed
- Domains:
- 3 x nucleotide binding domain (ATP) (21–25), (154–158), (171–187)
- Modification:
- phosphorylated on Arg-233 PubMed
- Cofactor(s): Mg2+
- Effectors of protein activity:
- Inhibited by citrate, PEP (Hill Coefficient 3) and Ca2+ (competes with Mg2+) in B. licheniformes PubMed.
- Inhibited by ATP (competitively) and f6p (non-competitively) in G. stearothermophillus PubMed
- Activated by GDP and ADP in lower concentrations (1mM); above that inhibition, competing with the ATP for the binding site (in G. stearothermophillus) PubMed
- Activated by NH4+ PubMed
- Localization: cytoplasm (Homogeneous) PubMed
Database entries
- BsubCyc: BSU29190
- Structure:
- UniProt: O34529
- KEGG entry: [3]
- E.C. number: 2.7.1.11
Additional information
- PfkA is a moonlighting protein. PubMed
- extensive information on the structure and enzymatic properties of PfkA can be found at Proteopedia
Expression and regulation
- Regulation:
- twofold induced by glucose PubMed
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- GP590 (pfkA::cat), available in Jörg Stülke's lab, PubMed
- GP595 (pfkA::erm), available in Jörg Stülke's lab, PubMed
- Expression vector:
- for expression/ purification from B. subtilis with N-terminal Strep-tag, for SPINE, in pGP380: pGP87, available in Jörg Stülke's lab
- for expression/ purification from B. subtilis with C-terminal Strep-tag, for SPINE, in pGP382: pGP1266, available in Jörg Stülke's lab
- for expression/ purification from E. coli with N-terminal His-tag, in pWH844: pGP393, available in Jörg Stülke's lab
- for expression in B. subtilis, in pBQ200: pGP1422, available in Jörg Stülke's lab
- lacZ fusion: pGP511 (in pAC6), available in Jörg Stülke's lab
- GFP fusion:
- two-hybrid system: B. pertussis adenylate cyclase-based bacterial two hybrid system (BACTH), available in Jörg Stülke's lab
- FLAG-tag construct: GP1019 (spc, based on pGP1331), available in Jörg Stülke's lab
- Antibody:
Labs working on this gene/protein
Jörg Stülke, University of Göttingen, Germany Homepage
Your additional remarks
References
Fabian M Commichau, Nico Pietack, Jörg Stülke
Essential genes in Bacillus subtilis: a re-evaluation after ten years.
Mol Biosyst: 2013, 9(6);1068-75
[PubMed:23420519]
[WorldCat.org]
[DOI]
(I p)
Alexander K W Elsholz, Kürsad Turgay, Stephan Michalik, Bernd Hessling, Katrin Gronau, Dan Oertel, Ulrike Mäder, Jörg Bernhardt, Dörte Becher, Michael Hecker, Ulf Gerth
Global impact of protein arginine phosphorylation on the physiology of Bacillus subtilis.
Proc Natl Acad Sci U S A: 2012, 109(19);7451-6
[PubMed:22517742]
[WorldCat.org]
[DOI]
(I p)
Joseph A Newman, Lorraine Hewitt, Cecilia Rodrigues, Alexandra S Solovyova, Colin R Harwood, Richard J Lewis
Dissection of the network of interactions that links RNA processing with glycolysis in the Bacillus subtilis degradosome.
J Mol Biol: 2012, 416(1);121-36
[PubMed:22198292]
[WorldCat.org]
[DOI]
(I p)
Martin Lehnik-Habrink, Joseph Newman, Fabian M Rothe, Alexandra S Solovyova, Cecilia Rodrigues, Christina Herzberg, Fabian M Commichau, Richard J Lewis, Jörg Stülke
RNase Y in Bacillus subtilis: a Natively disordered protein that is the functional equivalent of RNase E from Escherichia coli.
J Bacteriol: 2011, 193(19);5431-41
[PubMed:21803996]
[WorldCat.org]
[DOI]
(I p)
Martin Lehnik-Habrink, Henrike Pförtner, Leonie Rempeters, Nico Pietack, Christina Herzberg, Jörg Stülke
The RNA degradosome in Bacillus subtilis: identification of CshA as the major RNA helicase in the multiprotein complex.
Mol Microbiol: 2010, 77(4);958-71
[PubMed:20572937]
[WorldCat.org]
[DOI]
(I p)
María-Enriqueta Muñoz-Márquez, Elizabeth Ponce-Rivas
Effect of pfkA chromosomal interruption on growth, sporulation, and production of organic acids in Bacillus subtilis.
J Basic Microbiol: 2010, 50(3);232-40
[PubMed:20473954]
[WorldCat.org]
[DOI]
(I p)
Fabian M Commichau, Fabian M Rothe, Christina Herzberg, Eva Wagner, Daniel Hellwig, Martin Lehnik-Habrink, Elke Hammer, Uwe Völker, Jörg Stülke
Novel activities of glycolytic enzymes in Bacillus subtilis: interactions with essential proteins involved in mRNA processing.
Mol Cell Proteomics: 2009, 8(6);1350-60
[PubMed:19193632]
[WorldCat.org]
[DOI]
(I p)
Jean-Christophe Meile, Ling Juan Wu, S Dusko Ehrlich, Jeff Errington, Philippe Noirot
Systematic localisation of proteins fused to the green fluorescent protein in Bacillus subtilis: identification of new proteins at the DNA replication factory.
Proteomics: 2006, 6(7);2135-46
[PubMed:16479537]
[WorldCat.org]
[DOI]
(P p)
H Ludwig, G Homuth, M Schmalisch, F M Dyka, M Hecker, J Stülke
Transcription of glycolytic genes and operons in Bacillus subtilis: evidence for the presence of multiple levels of control of the gapA operon.
Mol Microbiol: 2001, 41(2);409-22
[PubMed:11489127]
[WorldCat.org]
[DOI]
(P p)
X Zhu, M Byrnes, J W Nelson, S H Chang
Role of glycine 212 in the allosteric behavior of phosphofructokinase from Bacillus stearothermophilus.
Biochemistry: 1995, 34(8);2560-5
[PubMed:7873536]
[WorldCat.org]
[DOI]
(P p)
M Byrnes, X Zhu, E S Younathan, S H Chang
Kinetic characteristics of phosphofructokinase from Bacillus stearothermophilus: MgATP nonallosterically inhibits the enzyme.
Biochemistry: 1994, 33(11);3424-31
[PubMed:8136379]
[WorldCat.org]
[DOI]
(P p)
C K Marschke, R W Bernlohr
Purification and characterization of phosphofructokinase of Bacillus licheniformis.
Arch Biochem Biophys: 1973, 156(1);1-16
[PubMed:4269800]
[WorldCat.org]
[DOI]
(P p)