Difference between revisions of "PdhA"
| Line 13: | Line 13: | ||
|- | |- | ||
|style="background:#ABCDEF;" align="center"|'''Function''' || links glycolysis and TCA cycle | |style="background:#ABCDEF;" align="center"|'''Function''' || links glycolysis and TCA cycle | ||
| + | |- | ||
| + | |colspan="2" style="background:#FAF8CC;" align="center"| '''Interactions involving this protein in [http://cellpublisher.gobics.de/subtinteract/startpage/start/ ''Subt''Interact]''': [http://cellpublisher.gobics.de/subtinteract/interactionList/2/PdhA PdhA] | ||
|- | |- | ||
|colspan="2" style="background:#FAF8CC;" align="center"| '''Metabolic function and regulation of this protein in [[SubtiPathways|''Subti''Pathways]]: <br/>[http://subtiwiki.uni-goettingen.de/pathways/carbon_flow.html Central C-metabolism]''' | |colspan="2" style="background:#FAF8CC;" align="center"| '''Metabolic function and regulation of this protein in [[SubtiPathways|''Subti''Pathways]]: <br/>[http://subtiwiki.uni-goettingen.de/pathways/carbon_flow.html Central C-metabolism]''' | ||
Revision as of 16:49, 30 July 2011
- Description: pyruvate dehydrogenase (E1 alpha subunit)
| Gene name | pdhA |
| Synonyms | aceA |
| Essential | yes |
| Product | pyruvate dehydrogenase (E1 alpha subunit) |
| Function | links glycolysis and TCA cycle |
| Interactions involving this protein in SubtInteract: PdhA | |
| Metabolic function and regulation of this protein in SubtiPathways: Central C-metabolism | |
| MW, pI | 41 kDa, 5.837 |
| Gene length, protein length | 1113 bp, 371 aa |
| Immediate neighbours | ykyA, pdhB |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 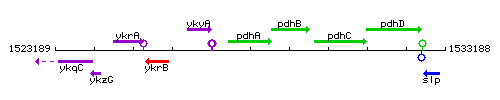
This image was kindly provided by SubtiList
| |
Contents
Categories containing this gene/protein
carbon core metabolism, essential genes
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU14580
Phenotypes of a mutant
- pdhA is essential PubMed
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: Pyruvate + [dihydrolipoyllysine-residue acetyltransferase] lipoyllysine = [dihydrolipoyllysine-residue acetyltransferase] S-acetyldihydrolipoyllysine + CO2 (according to Swiss-Prot)
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information: Michaelis-Menten PubMed
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Inhibited thiamine 2-thiothiazolone diphosphate and NADH PubMed
- Low sensibility to NADPH
- Localization:
Database entries
- Structure: 1W88 (E1 in complex with subunit binding domain of E2, Geobacillus stearothermophilus)
- UniProt: P21881
- KEGG entry: [3]
- E.C. number: 1.2.4.1
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
- stringent response: due to presence of guanine at +1 position of the transcript PubMed
- Additional information:
- The mRNA has a long 5' leader region. This may indicate RNA-based regulation PubMed
Biological materials
- Mutant:
- Expression vector:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Arthur Aronson, Purdue University, West Lafayette, USA homepage
Your additional remarks
References
Reviews
Kai Tittmann
Reaction mechanisms of thiamin diphosphate enzymes: redox reactions.
FEBS J: 2009, 276(9);2454-68
[PubMed:19476487]
[WorldCat.org]
[DOI]
(I p)
U Neveling, S Bringer-Meyer, H Sahm
Gene and subunit organization of bacterial pyruvate dehydrogenase complexes.
Biochim Biophys Acta: 1998, 1385(2);367-72
[PubMed:9655937]
[WorldCat.org]
[DOI]
(P p)
M S Patel, T E Roche
Molecular biology and biochemistry of pyruvate dehydrogenase complexes.
FASEB J: 1990, 4(14);3224-33
[PubMed:2227213]
[WorldCat.org]
[DOI]
(P p)
P A Frey
Mechanism of coupled electron and group transfer in Escherichia coli pyruvate dehydrogenase.
Ann N Y Acad Sci: 1982, 378;250-64
[PubMed:6805383]
[WorldCat.org]
[DOI]
(P p)
Original publications
Irnov Irnov, Cynthia M Sharma, Jörg Vogel, Wade C Winkler
Identification of regulatory RNAs in Bacillus subtilis.
Nucleic Acids Res: 2010, 38(19);6637-51
[PubMed:20525796]
[WorldCat.org]
[DOI]
(I p)
Shigeo Tojo, Kanako Kumamoto, Kazutake Hirooka, Yasutaro Fujita
Heavy involvement of stringent transcription control depending on the adenine or guanine species of the transcription initiation site in glucose and pyruvate metabolism in Bacillus subtilis.
J Bacteriol: 2010, 192(6);1573-85
[PubMed:20081037]
[WorldCat.org]
[DOI]
(I p)
Hans-Matti Blencke, Georg Homuth, Holger Ludwig, Ulrike Mäder, Michael Hecker, Jörg Stülke
Transcriptional profiling of gene expression in response to glucose in Bacillus subtilis: regulation of the central metabolic pathways.
Metab Eng: 2003, 5(2);133-49
[PubMed:12850135]
[WorldCat.org]
[DOI]
(P p)
Haichun Gao, Xin Jiang, Kit Pogliano, Arthur I Aronson
The E1beta and E2 subunits of the Bacillus subtilis pyruvate dehydrogenase complex are involved in regulation of sporulation.
J Bacteriol: 2002, 184(10);2780-8
[PubMed:11976308]
[WorldCat.org]
[DOI]
(P p)
M M Nakano, Y P Dailly, P Zuber, D P Clark
Characterization of anaerobic fermentative growth of Bacillus subtilis: identification of fermentation end products and genes required for growth.
J Bacteriol: 1997, 179(21);6749-55
[PubMed:9352926]
[WorldCat.org]
[DOI]
(P p)
P N Lowe, J A Hodgson, R N Perham
Dual role of a single multienzyme complex in the oxidative decarboxylation of pyruvate and branched-chain 2-oxo acids in Bacillus subtilis.
Biochem J: 1983, 215(1);133-40
[PubMed:6414463]
[WorldCat.org]
[DOI]
(P p)