Difference between revisions of "PckA"
| Line 33: | Line 33: | ||
<div align="right"> <small>This image was kindly provided by [http://genolist.pasteur.fr/SubtiList/ SubtiList]</small></div> | <div align="right"> <small>This image was kindly provided by [http://genolist.pasteur.fr/SubtiList/ SubtiList]</small></div> | ||
|- | |- | ||
| − | |colspan="2" |'''[http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=pckA_3129530_3131113_1 Expression at a glance]'''   {{PubMed|22383849}}<br/>[[Image:pckA_expression.png|500px]] | + | |colspan="2" |'''[http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=pckA_3129530_3131113_1 Expression at a glance]'''   {{PubMed|22383849}}<br/>[[Image:pckA_expression.png|500px|link=http://subtiwiki.uni-goettingen.de/apps/expression/expression.php?search=BSU30560]] |
|- | |- | ||
|} | |} | ||
Revision as of 15:22, 16 May 2013
- Description: phosphoenolpyruvate carboxykinase
| Gene name | pckA |
| Synonyms | ppc |
| Essential | no |
| Product | phosphoenolpyruvate carboxykinase |
| Function | synthesis of phosphoenolpyruvate |
| Gene expression levels in SubtiExpress: pckA | |
| Interactions involving this protein in SubtInteract: PckA | |
| Metabolic function and regulation of this protein in SubtiPathways: Central C-metabolism | |
| MW, pI | 58,1 kDa, 5.12 |
| Gene length, protein length | 1581 bp, 527 amino acids |
| Immediate neighbours | metK, ytmB |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 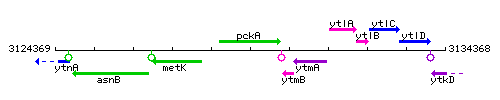
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed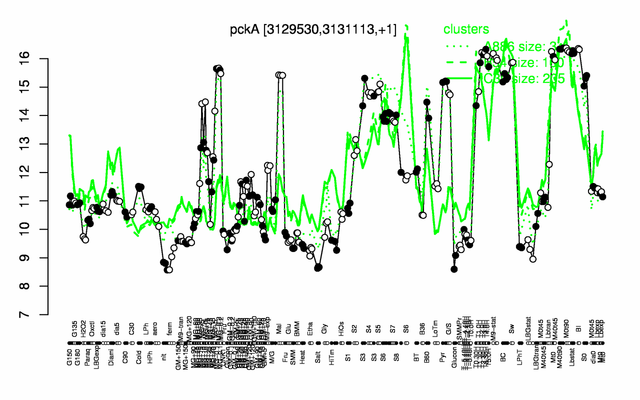
| |
Contents
Categories containing this gene/protein
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU30560
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: ATP + oxaloacetate = ADP + phosphoenolpyruvate + CO2 (according to Swiss-Prot) ATP + oxaloacetate = ADP + phosphoenolpyruvate + CO(2)
- Protein family: phosphoenolpyruvate carboxykinase [ATP] family (according to Swiss-Prot) phosphoenolpyruvate carboxykinase [ATP] family
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Nucleotide binding Domain (233–240)
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization: cytoplasm (according to Swiss-Prot), cytoplasm
Database entries
- Structure: 2PXZ (E.coli)
- UniProt: P54418
- KEGG entry: [3]
- E.C. number: 4.1.1.49
Additional information
Expression and regulation
- Additional information:
Biological materials
- Mutant:
- 1A1005 ( pckA::spec), PubMed, available at BGSC
- 1A996 ( pckA::spec), PubMed, available at BGSC
- GP1147 (pckA::neo), available in Jörg Stülke's lab
- Expression vector:
- pGP1753 (N-terminal Strep-tag, for SPINE, purification from B. subtilis, in pGP380) (available in Jörg Stülke's lab)
- pGP1762 (for expression, purification in E. coli with N-terminal His-tag, in pWH844, available in Jörg Stülke's lab)
- pGP1763 (for expression, purification in E. coli with N-terminal Strep-tag, in pGP172, available in Jörg Stülke's lab)
- lacZ fusion:
- two-hybrid system: B. pertussis adenylate cyclase-based bacterial two hybrid system (BACTH), available in Jörg Stülke's lab
- Antibody:
- FLAG-tag construct:
- GP1129 (spc, based on pGP1331), available in Jörg Stülke's lab
Labs working on this gene/protein
Stephane Aymerich, Microbiology and Molecular Genetics, INRA Paris-Grignon, France
Your additional remarks
References
Frederik M Meyer, Jörg Stülke
Malate metabolism in Bacillus subtilis: distinct roles for three classes of malate-oxidizing enzymes.
FEMS Microbiol Lett: 2013, 339(1);17-22
[PubMed:23136871]
[WorldCat.org]
[DOI]
(I p)
Matthew L Ferguson, Dominique Le Coq, Matthieu Jules, Stéphane Aymerich, Ovidiu Radulescu, Nathalie Declerck, Catherine A Royer
Reconciling molecular regulatory mechanisms with noise patterns of bacterial metabolic promoters in induced and repressed states.
Proc Natl Acad Sci U S A: 2012, 109(1);155-60
[PubMed:22190493]
[WorldCat.org]
[DOI]
(I p)
Frederik M Meyer, Jan Gerwig, Elke Hammer, Christina Herzberg, Fabian M Commichau, Uwe Völker, Jörg Stülke
Physical interactions between tricarboxylic acid cycle enzymes in Bacillus subtilis: evidence for a metabolon.
Metab Eng: 2011, 13(1);18-27
[PubMed:20933603]
[WorldCat.org]
[DOI]
(I p)
Simon Tännler, Eliane Fischer, Dominique Le Coq, Thierry Doan, Emmanuel Jamet, Uwe Sauer, Stéphane Aymerich
CcpN controls central carbon fluxes in Bacillus subtilis.
J Bacteriol: 2008, 190(18);6178-87
[PubMed:18586936]
[WorldCat.org]
[DOI]
(I p)
Pascale Servant, Dominique Le Coq, Stéphane Aymerich
CcpN (YqzB), a novel regulator for CcpA-independent catabolite repression of Bacillus subtilis gluconeogenic genes.
Mol Microbiol: 2005, 55(5);1435-51
[PubMed:15720552]
[WorldCat.org]
[DOI]
(P p)
Hans-Matti Blencke, Georg Homuth, Holger Ludwig, Ulrike Mäder, Michael Hecker, Jörg Stülke
Transcriptional profiling of gene expression in response to glucose in Bacillus subtilis: regulation of the central metabolic pathways.
Metab Eng: 2003, 5(2);133-49
[PubMed:12850135]
[WorldCat.org]
[DOI]
(P p)
Alia Lapidus, Nathalie Galleron, Alexei Sorokin, S Dusko Ehrlich
Sequencing and functional annotation of the Bacillus subtilis genes in the 200 kb rrnB-dnaB region.
Microbiology (Reading): 1997, 143 ( Pt 11);3431-3441
[PubMed:9387221]
[WorldCat.org]
[DOI]
(P p)