OpuAC
- Description: glycine betaine ABC transporter (binding protein)
| Gene name | opuAC |
| Synonyms | |
| Essential | no |
| Product | glycine betaine ABC transporter (binding protein) |
| Function | compatible solute transport |
| Metabolic function and regulation of this protein in SubtiPathways: Stress | |
| MW, pI | 32 kDa, 8.015 |
| Gene length, protein length | 879 bp, 293 aa |
| Immediate neighbours | opuAB, amhX |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 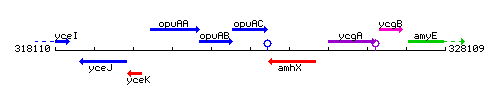
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU03000
Phenotypes of a mutant
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Interactions:
- Localization: cell membrane (according to Swiss-Prot)
Database entries
- Swiss prot entry: P46922
- KEGG entry: [2]
- E.C. number:
Additional information
Expression and regulation
- Operon:
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Erhard Bremer, University of Marburg, Germany homepage
Your additional remarks
References
Sander H J Smits, Marina Höing, Justin Lecher, Mohamed Jebbar, Lutz Schmitt, Erhard Bremer
The compatible-solute-binding protein OpuAC from Bacillus subtilis: ligand binding, site-directed mutagenesis, and crystallographic studies.
J Bacteriol: 2008, 190(16);5663-71
[PubMed:18567662]
[WorldCat.org]
[DOI]
(I p)
Carsten Horn, Stefan Jenewein, Linda Sohn-Bösser, Erhard Bremer, Lutz Schmitt
Biochemical and structural analysis of the Bacillus subtilis ABC transporter OpuA and its isolated subunits.
J Mol Microbiol Biotechnol: 2005, 10(2-4);76-91
[PubMed:16645306]
[WorldCat.org]
[DOI]
(P p)
Carsten Horn, Linda Sohn-Bösser, Jason Breed, Wolfram Welte, Lutz Schmitt, Erhard Bremer
Molecular determinants for substrate specificity of the ligand-binding protein OpuAC from Bacillus subtilis for the compatible solutes glycine betaine and proline betaine.
J Mol Biol: 2006, 357(2);592-606
[PubMed:16445940]
[WorldCat.org]
[DOI]
(P p)
Carsten Horn, Erhard Bremer, Lutz Schmitt
Functional overexpression and in vitro re-association of OpuA, an osmotically regulated ABC-transport complex from Bacillus subtilis.
FEBS Lett: 2005, 579(25);5765-8
[PubMed:16225868]
[WorldCat.org]
[DOI]
(P p)
Y Quentin, G Fichant, F Denizot
Inventory, assembly and analysis of Bacillus subtilis ABC transport systems.
J Mol Biol: 1999, 287(3);467-84
[PubMed:10092453]
[WorldCat.org]
[DOI]
(P p)
B Kempf, J Gade, E Bremer
Lipoprotein from the osmoregulated ABC transport system OpuA of Bacillus subtilis: purification of the glycine betaine binding protein and characterization of a functional lipidless mutant.
J Bacteriol: 1997, 179(20);6213-20
[PubMed:9335265]
[WorldCat.org]
[DOI]
(P p)
B Kempf, E Bremer
OpuA, an osmotically regulated binding protein-dependent transport system for the osmoprotectant glycine betaine in Bacillus subtilis.
J Biol Chem: 1995, 270(28);16701-13
[PubMed:7622480]
[WorldCat.org]
[DOI]
(P p)