Difference between revisions of "OpuAA"
| Line 66: | Line 66: | ||
=== Additional information=== | === Additional information=== | ||
| − | |||
=The protein= | =The protein= | ||
| Line 72: | Line 71: | ||
=== Basic information/ Evolution === | === Basic information/ Evolution === | ||
| − | * '''Catalyzed reaction/ biological activity:''' | + | * '''Catalyzed reaction/ biological activity:''' |
| + | ** uptake of glycine betaine | ||
| + | ** uptake of dimethylglycine {{PubMed|24561588}} | ||
* '''Protein family:''' [[ABC transporter]] domain (according to Swiss-Prot) | * '''Protein family:''' [[ABC transporter]] domain (according to Swiss-Prot) | ||
| Line 82: | Line 83: | ||
* '''Kinetic information:''' | * '''Kinetic information:''' | ||
| − | * '''Domains:''' | + | * '''[[Domains]]:''' |
* '''Modification:''' | * '''Modification:''' | ||
| − | * ''' | + | * '''[[Cofactors]]:''' |
* '''Effectors of protein activity:''' | * '''Effectors of protein activity:''' | ||
| Line 146: | Line 147: | ||
=References= | =References= | ||
| − | <pubmed>7622480,10092453,16645306,14623183,18321243,16225868, 21296969 22383849 23175650 23646920</pubmed> | + | <pubmed>7622480,10092453,16645306,14623183,18321243,16225868, 21296969 22383849 23175650 23646920 24561588</pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 14:48, 26 February 2014
- Description: glycine betaine ABC transporter (ATP-binding protein)
| Gene name | opuAA |
| Synonyms | |
| Essential | no |
| Product | glycine betaine ABC transporter (ATP-binding protein) |
| Function | compatible solute transport |
| Gene expression levels in SubtiExpress: opuAA | |
| Interactions involving this protein in SubtInteract: OpuAA | |
| Metabolic function and regulation of this protein in SubtiPathways: opuAA | |
| MW, pI | 46 kDa, 5.107 |
| Gene length, protein length | 1254 bp, 418 aa |
| Immediate neighbours | yceK, opuAB |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 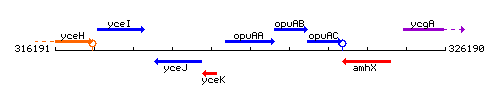
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
ABC transporters, coping with hyper-osmotic stress, membrane proteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU02980
Phenotypes of a mutant
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- uptake of glycine betaine
- uptake of dimethylglycine PubMed
- Protein family: ABC transporter domain (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Modification:
- Effectors of protein activity:
- Localization: associated to the membrane (via OpuAB) PubMed
Database entries
- Structure:
- UniProt: P46920
- KEGG entry: [2]
- E.C. number: 3.6.3.32
Additional information
Expression and regulation
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Erhard Bremer, University of Marburg, Germany homepage
Your additional remarks
References
Abdallah Bashir, Tamara Hoffmann, Sander H J Smits, Erhard Bremer
Dimethylglycine provides salt and temperature stress protection to Bacillus subtilis.
Appl Environ Microbiol: 2014, 80(9);2773-85
[PubMed:24561588]
[WorldCat.org]
[DOI]
(I p)
Jared T Winkelman, Anna C Bree, Ashley R Bate, Patrick Eichenberger, Richard L Gourse, Daniel B Kearns
RemA is a DNA-binding protein that activates biofilm matrix gene expression in Bacillus subtilis.
Mol Microbiol: 2013, 88(5);984-97
[PubMed:23646920]
[WorldCat.org]
[DOI]
(I p)
Tamara Hoffmann, Annette Wensing, Margot Brosius, Leif Steil, Uwe Völker, Erhard Bremer
Osmotic control of opuA expression in Bacillus subtilis and its modulation in response to intracellular glycine betaine and proline pools.
J Bacteriol: 2013, 195(3);510-22
[PubMed:23175650]
[WorldCat.org]
[DOI]
(I p)
Pierre Nicolas, Ulrike Mäder, Etienne Dervyn, Tatiana Rochat, Aurélie Leduc, Nathalie Pigeonneau, Elena Bidnenko, Elodie Marchadier, Mark Hoebeke, Stéphane Aymerich, Dörte Becher, Paola Bisicchia, Eric Botella, Olivier Delumeau, Geoff Doherty, Emma L Denham, Mark J Fogg, Vincent Fromion, Anne Goelzer, Annette Hansen, Elisabeth Härtig, Colin R Harwood, Georg Homuth, Hanne Jarmer, Matthieu Jules, Edda Klipp, Ludovic Le Chat, François Lecointe, Peter Lewis, Wolfram Liebermeister, Anika March, Ruben A T Mars, Priyanka Nannapaneni, David Noone, Susanne Pohl, Bernd Rinn, Frank Rügheimer, Praveen K Sappa, Franck Samson, Marc Schaffer, Benno Schwikowski, Leif Steil, Jörg Stülke, Thomas Wiegert, Kevin M Devine, Anthony J Wilkinson, Jan Maarten van Dijl, Michael Hecker, Uwe Völker, Philippe Bessières, Philippe Noirot
Condition-dependent transcriptome reveals high-level regulatory architecture in Bacillus subtilis.
Science: 2012, 335(6072);1103-6
[PubMed:22383849]
[WorldCat.org]
[DOI]
(I p)
Tamara Hoffmann, Erhard Bremer
Protection of Bacillus subtilis against cold stress via compatible-solute acquisition.
J Bacteriol: 2011, 193(7);1552-62
[PubMed:21296969]
[WorldCat.org]
[DOI]
(I p)
Carsten Horn, Stefan Jenewein, Britta Tschapek, Werner Bouschen, Sabine Metzger, Erhard Bremer, Lutz Schmitt
Monitoring conformational changes during the catalytic cycle of OpuAA, the ATPase subunit of the ABC transporter OpuA from Bacillus subtilis.
Biochem J: 2008, 412(2);233-44
[PubMed:18321243]
[WorldCat.org]
[DOI]
(I p)
Carsten Horn, Stefan Jenewein, Linda Sohn-Bösser, Erhard Bremer, Lutz Schmitt
Biochemical and structural analysis of the Bacillus subtilis ABC transporter OpuA and its isolated subunits.
J Mol Microbiol Biotechnol: 2005, 10(2-4);76-91
[PubMed:16645306]
[WorldCat.org]
[DOI]
(P p)
Carsten Horn, Erhard Bremer, Lutz Schmitt
Functional overexpression and in vitro re-association of OpuA, an osmotically regulated ABC-transport complex from Bacillus subtilis.
FEBS Lett: 2005, 579(25);5765-8
[PubMed:16225868]
[WorldCat.org]
[DOI]
(P p)
Carsten Horn, Erhard Bremer, Lutz Schmitt
Nucleotide dependent monomer/dimer equilibrium of OpuAA, the nucleotide-binding protein of the osmotically regulated ABC transporter OpuA from Bacillus subtilis.
J Mol Biol: 2003, 334(3);403-19
[PubMed:14623183]
[WorldCat.org]
[DOI]
(P p)
Y Quentin, G Fichant, F Denizot
Inventory, assembly and analysis of Bacillus subtilis ABC transport systems.
J Mol Biol: 1999, 287(3);467-84
[PubMed:10092453]
[WorldCat.org]
[DOI]
(P p)
B Kempf, E Bremer
OpuA, an osmotically regulated binding protein-dependent transport system for the osmoprotectant glycine betaine in Bacillus subtilis.
J Biol Chem: 1995, 270(28);16701-13
[PubMed:7622480]
[WorldCat.org]
[DOI]
(P p)