Difference between revisions of "OhrA"
| Line 121: | Line 121: | ||
* '''Additional information:''' | * '''Additional information:''' | ||
| + | ** number of protein molecules per cell (minimal medium with glucose and ammonium): 205 {{PubMed|24696501}} | ||
=Biological materials = | =Biological materials = | ||
Latest revision as of 10:01, 17 April 2014
- Description: peroxiredoxin , protects the cell against organic peroxides
| Gene name | ohrA |
| Synonyms | yklA |
| Essential | no |
| Product | peroxiredoxin |
| Function | organic peroxide resistance |
| Gene expression levels in SubtiExpress: ohrA | |
| MW, pI | 14 kDa, 5.061 |
| Gene length, protein length | 423 bp, 141 aa |
| Immediate neighbours | proA, ohrR |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 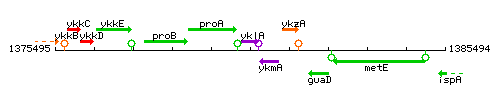
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed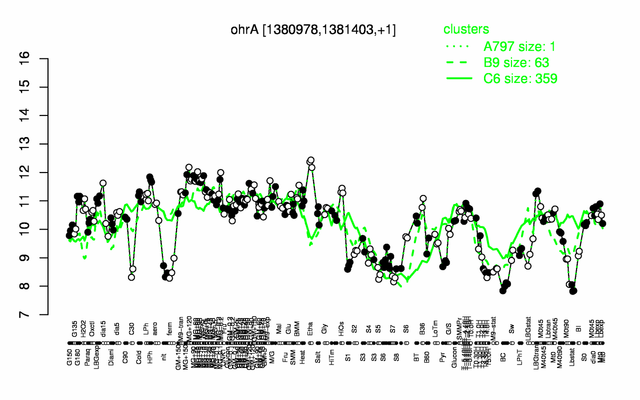
| |
Contents
Categories containing this gene/protein
resistance against oxidative and electrophile stress
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU13140
Phenotypes of a mutant
Database entries
- BsubCyc: BSU13140
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: osmC/ohr family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- BsubCyc: BSU13140
- Structure:
- UniProt: O34762
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Additional information:
- number of protein molecules per cell (minimal medium with glucose and ammonium): 205 PubMed
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Minsun Hong, Mayuree Fuangthong, John D Helmann, Richard G Brennan
Structure of an OhrR-ohrA operator complex reveals the DNA binding mechanism of the MarR family.
Mol Cell: 2005, 20(1);131-41
[PubMed:16209951]
[WorldCat.org]
[DOI]
(P p)
John D Helmann, Ming Fang Winston Wu, Ahmed Gaballa, Phil A Kobel, Maud M Morshedi, Paul Fawcett, Chris Paddon
The global transcriptional response of Bacillus subtilis to peroxide stress is coordinated by three transcription factors.
J Bacteriol: 2003, 185(1);243-53
[PubMed:12486061]
[WorldCat.org]
[DOI]
(P p)
M Fuangthong, S Atichartpongkul, S Mongkolsuk, J D Helmann
OhrR is a repressor of ohrA, a key organic hydroperoxide resistance determinant in Bacillus subtilis.
J Bacteriol: 2001, 183(14);4134-41
[PubMed:11418552]
[WorldCat.org]
[DOI]
(P p)
U Völker, K K Andersen, H Antelmann, K M Devine, M Hecker
One of two osmC homologs in Bacillus subtilis is part of the sigmaB-dependent general stress regulon.
J Bacteriol: 1998, 180(16);4212-8
[PubMed:9696771]
[WorldCat.org]
[DOI]
(P p)